Determination of Trans-Fatty Acid Levels in Selected Syrian Food Products
2025-01-23 | Volume 3 Issue 1 - Volume 3 | Research Articles | Radwan Badr Al-Deen | Bassam Al-Oklah | Mohamad Alshehabi | Tahani Al-ideeAbstract
Trans fatty acid (TFA) consumption correlates with negative health effects and contributes significantly to morbidity and mortality worldwide. The current study aimed to determine the levels of trans fatty acids (TFAs) and conjugated linoleic acid (CLA) in some Syrian food products to develop a TFAs composition database that would provide authorities with information on TFAS levels in Syrian food. In 2022, eighty-three samples were collected from Damascus city’s local market, including cow’s ghee (n=9), palm oil (n=7), sardine (n=9), olive oil (n=30, some of them are bottled in glass or plastic bottles and the others from local olive presses), soybean oil (n=7), sunflower oil (n=7), flaxseed oil (n=5), and sesame oil (n=2). The Soxhlet method was used to extract the lipids from the samples (except oils), then the methyl esters of fatty acids (FAME) were prepared and the concentration of fatty acids (g/100g) was determined using gas chromatography (GC) equipped with a Split/Splitless injector and a flame ionization detector (FID). The results revealed that the levels of Trans fatty acids (TFAs) ranged in all samples from 0.04 to 1.82 g/100g, with high amounts (4.78%) found in one sample of cow’s ghee, two samples of palm oil, and one sample of soybean oil. The olive oil, Flaxseed oil, sesame oil, and sardine contained less than 1 g TFAs/100g. Compared with the other samples, cow’s ghee showed the highest CLA concentration (0.60 g/100g). These findings suggest that additional reductions in Syria are required to properly meet public health objectives and lower the incidence of illnesses such as coronary heart disease.
Keywords : Trans Fatty Acids, Conjugated Linoleic Acid, Gas Chromatography, Syria.
INTRODUCTION
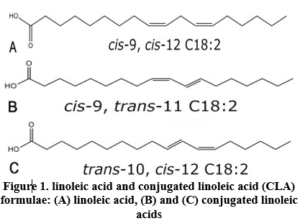
Trans fatty acids (TFAs) are unsaturated fatty acids with at least one double bond that is in the trans configuration. TFA are primarily derived from two sources: (1) ruminant trans fats, which occur naturally in dairy products and meat from ruminant animals; and (2) industrial trans fats, which are generated through the partial hydrogenation of vegetable oils [1]. During the thermal preparation of food, such as frying and baking, small amounts of TFA are also produced [2]. Trans isomers of oleic acid (18:1) are the most common in food products, followed by trans isomers of linoleic acid (18:2 n6), linolenic acid (18:3 n3), and palmitoleic acid (16:1). Cow’s ghee and partially hydrogenated fats have significantly different amounts and qualities of TFA [3]. Conjugated linoleic acid (CLA) is a polyunsaturated fatty acid present in animal fats such as red meat and dairy products [4]. Minimal amounts of CLA are present naturally in plant lipids, and various CLA isomers are generated via the chemical hydrogenation of fats (as illustrated in Figure 1). However, the CLA is not labeled as trans-fats [5].
Over the last three decades, there has been a growing amount of convincing scientific research on the health-damaging consequences of TFA [6]. TFA consumption has been linked to an increased risk of heart disease. TFA may increase the concentration of low-density lipoprotein (LDL) cholesterol while decreasing the concentration of high-density lipoprotein (HDL) cholesterol, both of which are risk factors for coronary heart disease [7]. According to the World Health Organization, TFA consumption should not exceed 1% of total daily energy intake (equal to less than 2.2 g/day in a 2000-calorie diet) [8]. The amount of trans fats in various food products and their daily intake in many countries has been estimated. TFA levels (g/100 g food) ranged from 0 to 0.246 in Argentina [9] and from 0 to 22.96 in India [10]. Ismail et al. [11] evaluated TFA in traditional and commonly consumed Egyptian foods and found that 34% of the products exceeded the TFA limit. Many Countries like Denmark, the United States, and Canada, have begun to reduce and eliminate trans fats in food through legislative initiatives that involved the implementation of regulations setting maximum limits of trans fats or mandated labeling of trans fats [12]. The United Arab Emirates is one of the leading Arab countries that have banned the presence of TFA in food products [13]. To our knowledge, no data on the trans fatty acids (TFAs) content of Syrian foods are available. Therefore, the current work’s objective is to give accurate and up-to-date information on the TFAs content of food products sold in Syria to create a steppingstone for the necessary laws and regulations to impose restrictions on the concentration of TFAs in imported or locally produced foods.
MATERIAL AND METHODS
Chemicals
TFA Standards, Hexan, Methanol, Sodium sulfate, Benzene, and Boron trifluoride were obtained from Merck (Germany). Fatty acids standard, namely: Methyl trans-9-Octadecenoate (elaidic acid methyl ester), Trans-11-Octadecenoic acid methyl ester (trans vaccenic acid methyl ester), Methyl cis-9-hexadecenoate (oleic acid methyl ester), linoleic acid methyl ester mix cis/trans, linolenic acid methyl ester isomer mix cis/trans, were purchased from Sigma-Aldrich (Germany).
Food samples
Seventy-six samples were collected from the local market of Damascus city in 2022. The samples included: Cow’s ghee (n=9), palm oil (n=7), Sardine (n=9), Olive oil (n=30), Soybean oil (n=7), Sunflower Oil (n=7), Flaxseed oil (n=5), and Sesame Oil (n=2).
Extraction of Fat
The Soxhlet method was used to extract the lipids from the samples (except oils) according to the method of AOAC (2019) [14]; briefly, 10 g of the sample were put into extraction thimble, which were extracted using 250 mL of N-Hexane, for 8 h. The extracted fat was used to prepare the methyl esters of fatty acids. The fats’ percentage in sardine samples were determined according to the Soxhlet method, but the fat which used in the preparation of FAME was extracted using a novel “cold method”, briefly, 10 g of the sardine sample was freeze-dried for 8 h. then the freeze-dried material was crushed manually using a porcelain pistol and mortar, transferred into airtight-screw cap glass bottle, soaked in 20 mL of N-Hexane, put in fridge (4°C) for 24 h. The N-Hexane (which containing the fat) was filtered, evaporated with Nitrogen current, and finely used to prepare FAMEs as described above. This novel method protected the polyunsaturated fatty acids (PUFAs), specially EPA and DHA from decomposition.
Fatty acids methyl esters preparation
The methyl esters of fatty acids (FAME) were prepared according to the procedure described by Morrison and Smith [15]. In a test tube, 0.02 g of fat was mixed with 2 ml of high-purity benzene and 2 ml of 7% BF3 in methanol. The tube’s vertical space was filled with nitrogen, closed tightly, and incubated in a boiling water bath (Memmert wb 14 models) for 60 minutes. After the mixture was cooled, 2 ml of n-hexane and 2 ml of water were added, and the tubes were centrifuged at 2000 rpm for 5 min. The supernatant was collected, transferred to a clean tube, mixed with 2 ml of water, and centrifuged again. The hexane layer was separated and mixed with anhydrous sodium sulphate, and 1μl was taken and injected into a gas chromatograph.
GC condition
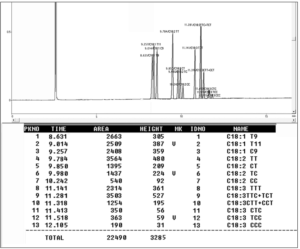
TFA was determined using a gas chromatograph (Shimadzu 17A, Japan) equipped with a Split/Splitless injector and a flame ionization detector (FID). A capillary column, PRECIX HP 2340 (60 m x 0.25 mm x 0.20 m film thickness), was used to separate and quantify each FAME component. The oven temperature program was set at 175 oC for 18 minutes, then the temperature was increased to 190oC at 5°C per minute, and the final temperature (190 oC) was maintained for 12 minutes. Nitrogen was used as the carrier gas at a flow rate of 1 mL/min. Methyl trans-9-Octadecenoate (elaidic acid methyl ester), Trans-11-Octadecenoic acid methyl ester (trans vaccenic acid methyl ester), Methyl cis-9-hexadecenoate (oleic acid methyl ester), linoleic acid methyl ester mix cis/trans (including CLAs, linoleic acid and trans linoleic acid), linolenic acid methyl ester isomer mix cis/trans, were identified (as shown in figure 1). The quantitative determination of trans fatty acid was calculated according to the peak areas.
RESULT
TFAs in cow’s ghee
The results in Table 1 showed that the total TFA in cow’s ghee ranged from 0.63 to 4.78 g/100 g. The results also showed that vaccenic acid was the major trans-18:1 isomer in the samples. Also, CLA with a concentration ranging from 0.21 to 1.35% was detected in cow’s ghee samples (Table 1).
TFAs in Olive Oil
The trans fatty acid content of the olive oil samples investigated is shown in Table 2 and varied between 0.0103 and 0.3349 g/100 g oil.
TFAs in Soybean, Sunflower, Flaxseed, and Sesame oils
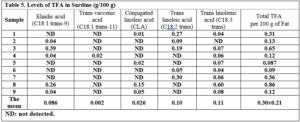
The Trans fatty acids content in soybean oil varied between 0.042 and 2.32 g/100g (Table 3). Table 3 shows the overall percentages (%) of TFA isomers detected in the sunflower oil samples tested. The TFA concentrations in the samples ranged from 0.13 to 1.23 g/100g. Table 3 also shows the distribution of TFAs in commercial flaxseed oil samples. The most common TFA isomers found in flaxseed oils were C18:3, followed by C18:2. While C18:1 trans-9, C18:1 trans-11, and CLA were not detected (as shown in Figure 3).
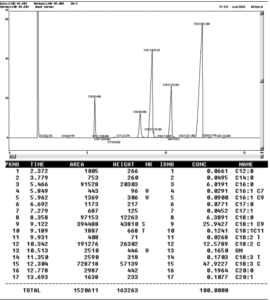
Table 3 illustrates the percentage of TFA in two examined sesame oil samples. The TFA concentrations observed in sesame oil ranged between 0.06 and 0.35 g/100 g (as shown in Figure 4)
TFAs in Sardine
TFA levels in sardine samples ranged from 0.087 to 0.75% (Table 5).
DISCUSSION
Cow’s ghee contains about 2.7% TFA with one or more trans-double bonds. Our results for TFAs in milk are consistent with the findings of Precht and Molkentin [16] and Vargas-Bello Pérez and Garnsworthy [17]. According to Shingfield et al [18], the trans-11 isomer is the main trans fatty acid in the group of trans-C18:1 isomers in cow’s ghee and represents about 40–50% of the total C18:1 trans fatty acids. Natural TFA, like vaccenic acid (18:1 t11), has anti-atherosclerotic and anti-diabetic properties [19]. The difference in vaccenic acid concentration between the samples investigated could be attributed to differences in the animal feeding system, which affects the amount of this isomer in cow’s ghee [20]. Dairy products contain the natural trans fatty acid conjugated linoleic acid (CLA), which has been linked to a lower risk of heart disease [21]. Vargas and Garnsworthy [17] confirmed that rumen bacteria can biohydrogenation unsaturated fatty acids and produce CLA. The variation in CLA concentration between samples is due to the difference in predominant microbial species and the diet offered to the animal [22]. All the olive oil samples studied were within the Syrian National Standard specification of olive oil (C18:1 T ≤0.05) [23], except for two samples for a total of C18:2 T + C18:3 T. This study’s results agree with the findings of Sakar et al. [24], who found that the trans fatty acid level of Moroccan olive oil was between 0.09 and 0.04 g/100 g fat. The total TFA content of Costa Rican and Egyptian olive oil samples is around 0.23 and 0.4 g/100 g, respectively [25] [11]. The amount of trans fatty acids in the Syrian olive oil samples varied, which may be related to differences in olive types, geographic area, and extraction methods. Sakar et al. [24] found a high level of TFA value in the super-pressure system, while the lowest one was displayed by the traditionally extracted oil, and the TFA correlated positively to K232, K270, and acid values. Our data revealed that the mean levels of TFA in soybean oil (1.0056%) were lower than the TFA concentration in Malaysian soybean oil (5.79%) [26]. The most frequent TFA in the soybean oil samples were C18:2 trans, C18:3 trans, and C18:1 trans-9. Soybean oil often contains more C18:2 trans isomers than C18:1 trans fatty acids, according to Aro et al. [27]. TFA in sunflower oils is probably generated by heating sunflower seeds before or during the extraction process [28]. Hou et al. [7] examined 22 samples of sunflower oil and found that the mean TFA was 1.41%, while Hoteit et al. [29] found a low concentration of TFA in sunflower oil samples (<0.1%) collected from the Lebanese market. C18:3 and C18:2 were the most common TFA isomers in flaxseed oils, which could be attributed to the lower levels of oleic and linoleic acids in linseed oil, which have been shown to be more stable than linolenic acid [30]. Bezelgues and Destaillats [31] reported that commercial linseed oil for human consumption is refined; during the refining process, TFA forms due to high amounts of unsaturated fatty acids (UFA) and high temperatures, particularly during the deodorization step. According to Johnson et al. (2009) [32], the total TFA content in Indian sesame oil was 1.3%, and Elaidic acid comprised the majority. Sesame oil’s TFA in the Malaysian market ranged from 0.1 to 0.76%. While Song et al. (2015) [33] did not detect any TFA in Korean sesame oil. The presence of TFA in refined palm oils is due to thermal isomerization caused by the relatively high temperatures of up to 260°C used during deodorization [34]. According to Hishamuddin et al. [6], TFA levels in Malaysian palm oil range between 0.24 and 0.67 g/100g. Wolff [35] has previously demonstrated that TFA formation is strongly influenced by heating time and deodorization temperature; thus, longer deodorization times at higher temperatures may increase the TFA content of refined oils. TFA was also detected at low levels in frozen sardine samples (Table 5). Nasopoulou et al. [36] found the levels of C18:1 trans (ω-9) in raw, grilled, and brined sardines to be 39.7, 65.3, and 96.7 mg/kg fish tissue, respectively.
CONCLUSION
The study’s findings indicated that TFA of natural origin (Vaccenic acid) could be differentiated from TFA of industrial origin (Elaidic acid) in the products analyzed. We found that cow’s ghee contained the highest percentages of CLA, which is known to have health advantages. Olive, sesame, and flaxseed oils are healthy oils that have low levels of TFA. Future studies will focus on the levels of TFA in other food products, especially chocolate bakery products, fast food consumed, and foods common in the Syrian local market.
References :- Lottenberg AM. Importance of the dietary fat on the prevention and control of metabolic disturbances and cardiovascular disease. Arquivos Brasileiros de Endocrinologia & Metabologia. 2009;53:595-607.
- Wanders AJ, Zock PL, Brouwer IA. Trans fat intake and its dietary sources in general populations worldwide: a systematic review. Nutrients. 2017;9(8):840.
- Precht D, Molkentin J. Trans fatty acids: implications for health, analytical methods, incidence in edible fats and intake. Food/Nahrung. 1995;39(5-6):343-74.
- Gonçalves DC, Lira FS, Carnevali LC, Rosa JC, Pimentel GD, Seelaender M. Conjugated Linoleic Acid: good or bad nutrient. Diabetology & Metabolic Syndrome. 2010;2:1-2.
- Basak S, Duttaroy AK. Conjugated linoleic acid and its beneficial effects in obesity, cardiovascular disease, and cancer. Nutrients. 2020;12(7):1913.
- Hishamuddin E, Abd Razak RA, Yeoh CB, Ahmad Tarmizi AH. Assessment of trans fatty acid levels in refined palm-based oils and commercial vegetable oils in the malaysian market. J. Oil Palm Res. 2022;34:129-38.
- Hou JC, Wang F, Wang YT, Xu J, Zhang CW. Assessment of trans fatty acids in edible oils in China. Food Control. 2012;25(1):211-5.
- World Health Organization. Countdown to 2023: WHO report on global Trans-fat elimination 2022. World Health Organization; 2023.
- Monge-Rojas R, Vargas-Quesada R, Chinnock A, Colón-Ramos U. Changes in dietary intake of major nutrients and food sources among Costa Rican adolescents in the last 20 years. The Journal of Nutrition. 2020;150(9):2405-11.
- Gupta V, Downs SM, Ghosh-Jerath S, Lock K, Singh A. Unhealthy fat in street and snack foods in low-socioeconomic settings in India: a case study of the food environments of rural villages and an urban slum. Journal of nutrition education and behavior. 2016;48(4):269-79.
- Ismail G, Abo El Naga R, El Sayed Zaki M, Jabbour J, and Al-Jawaldeh, A. Analysis of fat content with special emphasis on trans isomers in frequently consumed food products in Egypt: the first steps in the trans fatty acid elimination roadmap. Nutrients, (2021);13(9), 3087.
- Zuchowska-Grzywacz M, Kowalska M. Trans fatty acids in food–current legal regulations as protections for consumers and food manufacturers. Acta Alimentaria. 2019;48(1):105-14.
- Nagpal T, Sahu JK, Khare SK, Bashir K, Jan K. Trans fatty acids in food: A review on dietary intake, health impact, regulations and alternatives. Journal of Food Science. 2021;86(12):5159-74.
- Association of Official Analytical Chemists. Official Methods of Analysis, 21st; (2019). Association of Official Analytical Chemists: Washington, DC, USA.
- Morrison WR, Smith LM. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride–methanol. Journal of lipid research. 1964;5(4):600-8.
- Precht D, Molkentin J. Trans fatty acids: implications for health, analytical methods, incidence in edible fats and intake. Food/Nahrung. 1995;39(5-6):343-74.
- Vargas-Bello Pérez E, Garnsworthy PC. Trans fatty acids and their role in the milk of dairy Ciencia e investigación agraria. 2013;40(3):449-473.
- Shingfield KJ, Chilliard Y, Toivonen V, Kairenius P, Givens DI. Trans fatty acids and bioactive lipids in ruminant milk. Bioactive components of milk. 2008;1:3-65.
- Prandini A, Sigolo S, Piva G. A comparative study of fatty acid composition and CLA concentration in commercial cheeses. Journal of Food Composition and Analysis. 2011;24(1):55-61.
- Dhiman TR, Nam SH, Ure AL. Factors affecting conjugated linoleic acid content in milk and meat. Critical reviews in food science and nutrition. 2005;45(6):463-82.
- Bonthuis M, Hughes MC, Ibiebele TI, Green AC, Van Der Pols JC. Dairy consumption and patterns of mortality of Australian adults. European journal of clinical nutrition. 2010;64(6):569-77.
- Lee YJ, Jenkins TC. Biohydrogenation of linolenic acid to stearic acid by the rumen microbial population yields multiple intermediate conjugated diene isomers. The Journal of Nutrition. 2011;141(8):1445-50.
- SNS. Olive oil. (SNS 182:2016).Syrian National standard, 2016.
- Sakar EH, Khtira A, Aalam Z, Zeroual A, Gagour J, Gharby S. Variations in physicochemical characteristics of olive oil (cv ‘Moroccan Picholine’) according to extraction technology as revealed by multivariate analysis. AgriEngineering. 2022;4(4):922-38.
- Baylin A, Siles X, Donovan-Palmer A, Fernandez X, Campos H. Fatty acid composition of Costa Rican foods including trans fatty acid content. Journal of Food Composition and Analysis. 2007;20(3-4):182-92.
- Akmar ZD, Norhaizan ME, Azimah R, Azrina A, Chan YM. The trans fatty acids content of selected foods in Malaysia. Malaysian Journal of Nutrition. 2013;19(1).
- Aro A, Van Amelsvoort J, Becker W, van Erp-Baart MA, Kafatos A, Leth T, Van Poppel G. Transfatty acids in dietary fats and oils from 14 European countries: the TRANSFAIR study. Journal of Food Composition and Analysis. 1998;11(2):137-49.
- Tasan M, Demirci M. Trans FA in sunflower oil at different steps of refining. Journal of the American Oil Chemists’ Society. 2003;80(8):825-8.
- Hoteit M, Zoghbi E, Rady A, Shankiti I, Ibrahim C, Al-Jawaldeh A. Non-Conjugated-Industrially-Produced-Trans Fatty in Lebanese Foods: The Case of Elaidic and Linolelaidic Acids. Nutrients. 2021;13(10):3664.
- Tsuzuki W, Nagata R, Yunoki R, Nakajima M, Nagata T. cis/trans-Isomerisation of triolein, trilinolein and trilinolenin induced by heat treatment. Food chemistry. 2008;108(1):75-80.
- Bezelgues JB, Destaillats F. Formation of trans fatty acids during deodorization of edible oils. InTrans fatty acids in human nutrition 2012; (pp. 65-75). Woodhead Publishing.
- Johnson S, Saikia N, Mathur HB, Agarwal HC. Fatty acids profile of edible oils and fats in India. Centre for Science and Environment, New Delhi. 2009:1-49.
- Song J, Park J, Jung J, Lee C, Gim SY, Ka H, Yi B, Kim MJ, Kim CI, Lee J. Analysis of trans fat in edible oils with cooking process. Toxicological research. 2015;31:307-12.
- Sue, T. T.(2002). Fatty acid composition of edible oils in the Malaysian markets, with special reference to the trans-fatty acids. Journal of Oil Palm Research, 14(1), 1-8.
- Wolff RL. Further studies on artificial geometrical isomers of α-linolenic acid in edible linolenic acid-containing oils. Journal of the American Oil Chemists’ Society. 1993;70:219-24.
- Nasopoulou C, Psani E, Sioriki E, Demopoulos CA, Zabetakis I. Evaluation of sensory and in vitro cardio protective properties of sardine (Sardina pilchardus): The effect of grilling and brining.
The authors declare there is no competing interest
Fund: No Fund
Author contributions: All authors contributed equally.
Compliance with Ethical Standards: This article does not contain any studies involving human or animal subjects.
Data and materials availability: All data and materials are available in the main text.
(ISSN - Online)
2959-8591