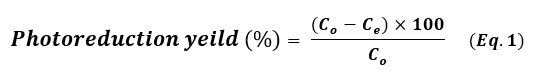Photo-assisted catalytic reduction performance of three noble metal nanoplatforms (Ag NPs, Pt NPs, Au NPs) and its correlation with the heterostructural properties: Probe sonication fabrication
2025-07-21 | Volume 3 Issue 2 - Volume 3 | Research Articles | Muna Qareh | Sadra Alsaid | Maya Zanouba | Gheffar KaraAbstract
In this study, three types of noble metal nanocatalysts (Pt NPs, Au NPs, and Ag NPs) were prepared using a green and environmentally safe method – ultrasound probe method – based on the polyol approach. The reduction of metal ions was carried out in two reduction stages: simple and strong reduction. Physicochemical characterization was carried out using a set of relevant and important techniques. The results of the crystal phase study revealed that the pure crystal phases of the three catalyst particles belong to the FCC system, with accurate crystallographic parameters (crystallite size, crystallinity degree, and orientation degree). The results of the aforementioned study were consistent with the results of the elemental analysis. The investigation of these parameters confirmed that the Pt and Ag nanocatalyst particles were built from perfectly crystalline nuclei. While the Au nanocatalyst particles were unique in their crystalline characteristics, demonstrating that their crystal structure was formed from primary crystalline nuclei, unlike the other two catalyst particles. Not only did the three nanocatalyst particles share spherical morphologies, but the Au and Pt nanocatalyst particles also possessed polygonal and sheet morphologies, unlike Ag NPs. To exploit these structural differences, the photoreduction catalytic performance of them was determined in the presence of sodium borohydride on two groups of contaminants: the first group, colored-based contaminants (methylene blue and rhodamine B), and the second, petroleum-based contaminants (p-nitrophenol and trichlorobenzene) at different concentrations. The catalytic yields of the colored contaminants were significantly higher than those of the petroleum-based contaminants. Ag NPs exhibited the highest photoreduction catalytic potential compared to Pt NPs and Au NPs. After conducting reusability tests for each nanocatalyst individually, the green property of all three nanocatalysts demonstrated that they can be reused multiple times and offer superior performance.
Keywords : Noble Elements, Nanocatalysts, Green Protocol, Crystalline Nuclei, Distinctive Morphology, Methylene Blue, Rhodamine B, P-Nitrophenol, Trichlorobenzene, Photoreduction Catalytic Peformance.
INTRODUCTION
Recently, a growing number of warnings have been issued about the fate of life on planet Earth. Its ecosystem, with all its components, has been constantly polluted -both organic and inorganic- due to the combination of the spread of industrial revolutions and the increase in various human activities. Revolutions in the textile and oil industries have had a great potential to cause terrible and catastrophic deterioration of the aquatic environment, and they did what they did in the past and what these deteriorations have led to today (1). In several Asian and African countries, as a result of population growth and to secure greater economic returns for the country, governments have turned the wheel of production to expand textile, medical, pesticide, and other industries as a central tributary to strengthening and growing their economies. But the development rewards have not been good with regarding the production of organic dyes and petroleum-based pesticides. Production has reached immeasurable levels – tens of thousands of tons of dyes, pesticides, and even raw materials for medicines – causing negative impacts on rivers, drinking water sources, and brackish water sources (1,2). Not all scientific studies have concealed the fact that the gradual accumulation of byproducts from that production process (heavy metals, fillers, lubricants for production equipment, etc.) is being introduced as non-biodegradable waste into water, exposing water bodies to potentially unavoidable hazards in the future. The strong interconnectedness of the living and non-living components of water and the ease of movement of organic contaminants between them increases the complexity of water pollution and the interconnection of this pollution with other media. These contaminants affect light penetration in water, impair the formation of chlorophyll in aquatic plants, increase the rate of anaerobic fermentation, cause the death of marine organisms, decrease the levels of important ions (potassium, sodium, chloride, etc.), and other effects caused by water pollution with organic matter. Organic contaminants fall into different families, classified according to their toxicity or chemical composition: colored azo contaminants (homogeneous and heterocyclic aromatic compounds), petroleum contaminants (monocyclic aromatic compounds), and dozens of dirty groups of hydrocarbon compounds (POPs and PAHs), etc. (3). This group, out of one hundred and twenty-nine known priority contaminants, as described in the U.S. Clean Water Act, silently depletes and kills aquatic resources with dire consequences. This consequence falls within the scope of the characteristics of persistent organic contaminants, including: accumulating capacity, complexity of chemical composition, heterogeneous and unstable distribution between solid and liquid phases, high solubility in lipids, and bioaccumulation in human and animal tissues (4-6). According to the reports of researchers Al-Tohamy (1), Bishnoi (2), and Abu-Nada (7), phenolic hydrocarbons and halogenated monoaromatic hydrocarbons, commonly used as pesticides, are highly enriched compared to polycyclic hydrocarbons in agricultural soils, wastewater, and industrial sludge. Researchers’ conclusions regarding the reason behind this enrichment have converged (1) (2) (7). Their conclusions regarding the increased enrichment in soils and wastewater media were as follows: phenolic and halogenated compounds can interact with available organic compounds through an adsorption mechanism (8). Over the past few decades of the last century and the current one, the lofty goal of preserving the environment’s water resources, in the first place, and other environments in the second place, has been a constant preoccupation and major concern for researchers. This has been highlighted by the submission and publication of thousands of quality articles aimed at finding ways to treat water from various forms and types of organic contaminants. Given the unresponsiveness of complex contaminants to environmental degradation – accomplished through chemical or biological reactions – and the antiquated nature of previously designed treatment methods, technology researchers have emphasized the creating of interdisciplinary collaboration environment between chemistry and environmental science to generate brighter and more qualitative solutions for water treatment. After continuous research and arduous experiments, this alliance has resulted in the development of a new generation of ultra-small materials – so-called nanomaterials – in various polymeric, metallic, and organic forms, using modern and sustainably developed methodologies. Nanoscale researchers are obsessed with using metallic compounds (primarily noble metal nanoparticles) with their excellent optical/magnetic/structural/crystalline/surface properties to neutralize a significant portion of organic contaminants in water. Many methods based on composites/hybrids/alloys of small-sized noble metal particles have been proposed for contaminant removal, including precipitation, coagulation, adsorption, and others (9). The photoreduction method relies on the presence of different light sources (infrared, ultraviolet, visible light) and relies on photoactive materials such as Cd-MOF (10), zinc oxide (11), cadmium sulfide (13), and zero-valent iron nanoparticles (14). This method is characterized by its economy, ease of application, and low environmental side effects. The basic premise of the photoreduction mechanism revolves around two fundamental points: the first is the change in the bandgap value of the nanoscale catalyst with degradation ability, and the second is the surface plasmon resonance (SPR) property. Regarding the first point, two different semiconductors, p and n, must be available to generate a continuous cascade of electron-hole pairs. Regarding the second point, this property arises from the collective movement of free electrons localized on the surface of nanoparticles (especially gold, silver, copper, and platinum “to a lesser extent”) when light falls on them (15). Due to their thermal stability, chemical inertness to oxidizing agents, bioactivity, unique surface properties, and the possibility of generating them at nanoscale and in various morphologies, noble metal particles (Pd, Rh, Au, Ag, Pt) have attracted the attention of many biological researchers, chemists, and bioengineers in many applications (16) (17) (18). For their part, researchers interested in environmental cleanliness and preserving it from any imminent danger are increasingly developing methods for using these metals in environmental applications such as advanced oxidation of organic compounds, reducing the effects of toxic gas emissions from internal combustion engines in transportation, water splitting, and more (19). Many researchers have utilized noble metal nanoparticles in the catalytic reactions of organic compounds (dyes, petroleum derivatives, pesticides) – after loading them onto the surface of metal oxides such as titanium oxide, zinc oxide, copper ferrite, etc., or applying harsh reaction conditions – to increase catalytic activity and accelerate contaminant removal (5) (10) (11). Liu presented a paper on the effect of crystallization on the catalytic performance of titanium oxide supported by gold particles (16). Liu found that the improved crystalline properties with the presence of gold particles favorably accelerated the catalytic degradation process of a number of contaminants (16). In another paper by Zheng et al., it was demonstrated that the combination of zinc oxide with silver zero-valent “Ag(0)” resulted in a positive improvement in electron-hole generation, which in turn improved the degradation performance of the nanostructure under visible light irradiation (20). In Zheng’s paper (20), the synthesis of three metallic nanoparticles using ultrasonication in a weakly alkaline medium and in the presence of sodium borate tetrahydride was reported. Each metallic nanoparticle was characterized by its own nanoscale structure (morphology and crystallography). This study aimed to establish the foundations of green chemistry, particularly by utilizing the probe-ultrasound method to prepare three different nanoscale catalysts (Ag NPs, Au NPs and Pt NPs) under safe and easy-to-use conditions. The different structural properties that resulted from their characterization paved the way for their applicability in catalytic reactions using a simulated sunlight source (in the visible range “λ= 200-800 nm”). Crucially, these differences in properties led to a tangible comparative study between the catalytic decomposition results of the four contaminants, p-NP, MB, TCB, and Rh B. The novelty presented in this research is the environmental sustainability of the prepared particles, as these nanoparticles can be reused multiple times with high efficiency. Ag NPs demonstrated the highest photoreduction catalytic performance in removing all contaminants from aqueous media at all applied concentrations. Pt NPs ranked second in the photoreduction reaction, followed by Au NPs. The photoreduction behaviors differed with the contaminant type. The excellent reusability rates evinced clearly that the three groups of prepared particles are efficient for future photoreduction applications.
MATERIALS AND METHODS
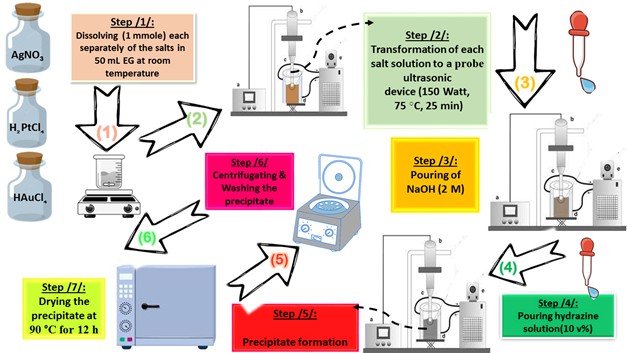
All chemicals, as listed below, used in this paper were supplied from Sigma-Aldrich (China) without further purification: HAuCl4. 3H2O (≥ 99.99%, Au basis), H2PtCl6.6H2O (≥ 37.50%, Pt basis), AgNO3 (≥ 99.00%, trace metal basis), ethylene glycol (EG) ((CH2)2(OH)2 anhydrous, 99.8%), hydrazine (N2H4.H2O, 80.00%), methylene blue (MB, C16H18ClN3S · xH2O, ≥95.00 %), para-nitrophenol (p-NP, O2NC6H4OH, ≥99.00%), 2,4,6-tricholrobenzene (TCB, Cl3C6H2SO2Cl, ≥96.00 %) and Rhodamine B (Rh B, C28H31ClN2O3, ≥ 95.00 %). In order to prepare the different photocatalysts considered in this paper (Pt NPs, Au NPs and Ag NPs), a suitable molar ratio of each metal precursor (“0.13653 g (HAuCl4.3H2O)”, “0.13725 g (H2PtCl6.6H2O)”, “0.75295 g (AgNO3)”) was mixed with 50 mL of EG in three separate beakers. The solution was heated at 75 °C for four hours with gentle magnetic stirring, observing the initial color change (in the Au3+/EG solution from yellow to very dark gold, in the Pt4+/EG solution from intense orange to orange-brown, in the Ag+/EG solution from transparent to pale gray). Then, the glass beakers were transferred to an ultrasonic probe system (sono-horn made of titanium metal, 12.5 mm in diameter, operating at 20 kHz with a maximum power output of 600 W). Each solution was sonicated according to the following profile (300 sec “on”, 120 sec “off”, at 75 °C, time sonication of 25 min, 150 Watt). During sonication, sodium hydroxide solution (2 M) was added until the pH of the medium became 12, then 5 ml of hydrazine solution (10% v/v) was added dropwise. The colors of the formed precipitates were as follows: black (in the case of Pt NPs), dark brown (in the case of Au NPs) and dark gray (in the case of Ag NPs). Each precipitate was washed several times with a mixture of ultrapure water/ethanol (1:2 v/v) to remove any remaining unreacted materials. In the final stage, each precipitate was dried at 90 °C for 12 h. Figure 1. represents the schematic of the preparation stages by probe sonication of nanoscale particles based on noble metals (Pt NPs, Au NPs and Ag NPs).
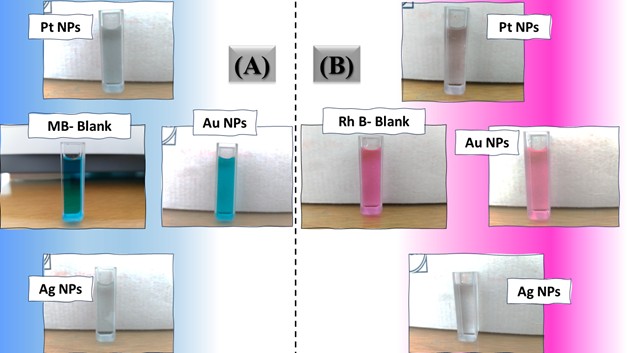
The photoreduction catalytic reaction of four hazardous organic pollutants – methylene blue (MB), para-nitrophenol (p-NP), Rhodamine B (RhB), and 2,4,6-trichlorobenzene (TCB) – in the presence of NaBH₄ under visible light irradiation was employed as a model photoreduction catalytic reaction to evaluate the reduction catalytic performance of the synthesized noble metal nanoparticles (Pt NPs, Au NPs, and Ag NP). A NaBH4 solution 0.26 Mm was prepared and stored in the dark. In a typical photoreduction test of the contaminants, 10.00 mg of the nano-catalyst (Pt NPs, Au NPs and Ag NPs) was poured separately into the aqueous solution of the related contaminant (10 mL, 10 mg.L-1 “ppm”), then ultrasonicated at room temperature for 60 sec. 100 μL of NaBH4 aqueous solution (0.26 mM) was mixed with the contaminant solution. After sonication, the solutions were exposed to visible light for three continuous hours. 5 mL of suspension – containing both the photocatalyst and the target contaminant – was taken out and centrifuged at 6000 rpm. All irradiations were performed using a white LED lamp (the radiant intensity (3 mw/cm2) in the wavelength range 400-780 nm with 10% of this in the ultraviolet range, and power density of 7-10 W at 0.0083 A, optical rising time 7 ns, intensity of the illumination 400 µW.cm-1 and ≥ 10 mm of diameter) as a solar-simulated light source. The photoreduction outcomes were read using a UV-Vis. spectrophotometer and using the Beer-Lambert law at a prominent wavelength for each contaminant solution (λ=664 nm for MB, λ=405 nm for p-NP, λ=555 nm for Rh B and λ=265 nm for TCB), which corresponded to the maximum absorbance of the contaminant mother solution. Dye uptake can also quantified using the efficiency of dye photocatalysis given by using the following equation 1:
Where, Co is the initial concentration of the contaminant solution in terms of mg.L-1 and Ce is the equilibrium concentration of the contaminant solution in terms of mg.L-1. The photoreduction efficiency of contaminants from their aqueous solutions depends strongly on the initial concentration. In order to assess, different concentrations of each contaminant (5, 10 ,15 and 20 mg.L-1) were tested at pH 7 with 10 mg of each nanocatalyst added into 10 mL solutions at 20 ˚C. The level of catalyst reusability plays an important role in these applications. After each catalysis cycle, for the first time, the nano-catalyst was separated from the reaction by centrifugation, washed with ultrapure water/ethanol, and then dried at 90 ° C for 12h (21). The applied conditions of the photoreduction reaction are summarized in Table 1.
Powder X-ray diffraction (PXRD) measurements were implemented using X’ pert pro. Analytical company with Cu-Kα radiation (λ= 1.5406 Å, scanning rate of 0.02 θ·s⁻¹, operating at 40 kV and 40 mA) to determine the crystal phases of the nanocatalysts. Field emission scanning electron microscopy (FESEM) with an accelerating voltage of 3 kV (MAIA3, TESCAN, Czech Republic) and transmission was applied to examine the morphology/size of the nanocatalysts. Energy Dispersive Spectroscopy (EDS) analysis was acquired by a “MAIA3, TESCAN” at the 15 kV acceleration voltages. The internal structure morphology of the (Pt NPs, Au NPs, Ag NPs) and the variation of the concentrations of the colored solutions of the contaminants were studied using TEM images (model Zeiss-EM10C-100KV, operating at an accelerating voltage of 160 kV) and dual-band UV-Vis. Spectroscopy in quartz cells (Shimadzu, mini 1240 (UV), in the wavelength range of 200-800 nm).
RESULTS
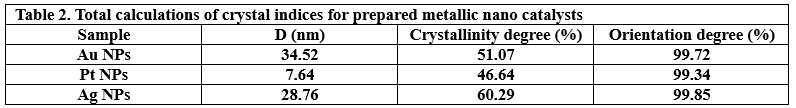
The crystalline state of Ag NPs, Au NPs and Pt NPs was examined by the X-ray diffraction patterns (Figure 2.). The diffraction peaks observed for the prepared Ag NPs were related to the following Miller indices (1 1 1), (0 0 2), (0 2 2), (1 1 3) and (2 2 2), which were located at diffraction angles of 38.12°, 44.39°, 64.54°, 77.49° and 81.6°. According to what this pattern showed and its comparison with many related references (17) (22) (23), it is clear that the Ag NPs were associated with the reference card Ag NPs (JCPDS-04-0783). On the other hand, as shown in the XRD pattern in Figure 2., for Au NPs, five diffraction peaks can be observed located at diffraction angles of 38.18°, 44.43°, 64.87°, 77.78° and 82.22°, which were related to Miller indices (1 1 1), (0 0 2), (0 2 2), (1 1 3) and (2 2 2), respectively. The characteristic diffraction pattern of Au was referenced in JCPDS card no. 04-0784 (17). The XRD pattern (Figure 2.) showed that Pt NPs main peaks were observed at 39.80°, 46.01°, 67.35° and 88.60°, which were almost identical to the reference card for Pt NPs (JCPDS 04-0802) (17). Thus, the reduction of silver, gold and platinum ions and the production of pure samples without impurities were confirmed. The crystal grain sizes, degree of crystallinity and orientation degree of those were calculated by the corresponding equations, which were reported in many papers (17) (18) (21), as shown in Table 2.
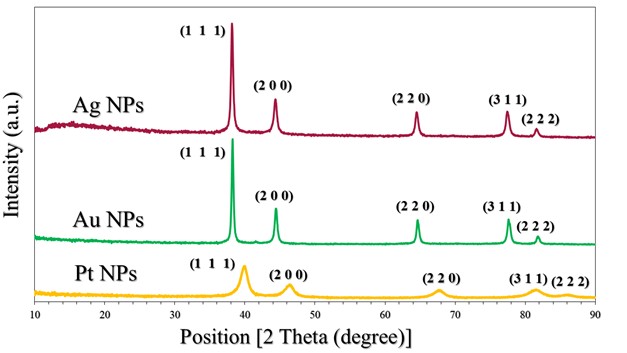
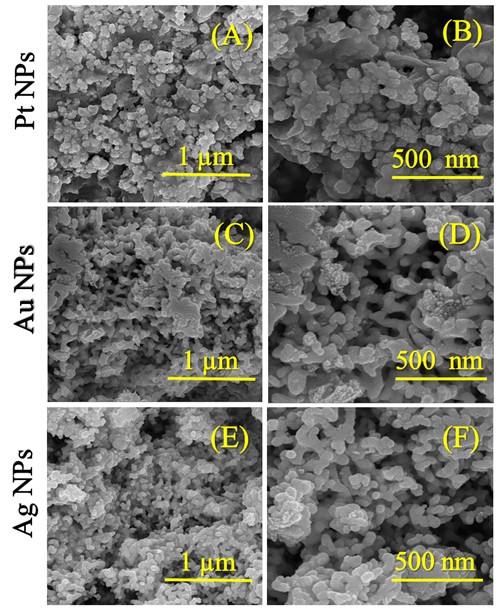
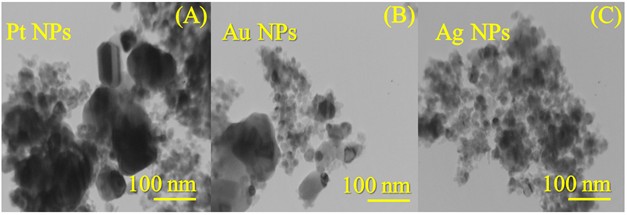
Figure 3.(A-F) presents the FESEM micrographs of the synthesized nanocatalyst particles. As disclosed in Figure .3(A&B), the Pt NPs had the shape of small cauliflower buds with slightly rough surfaces (see supplementary material file in Figure S1.(A&C)) and a small spherical-like shape with an average size of 28.71 nm. Some sheets were also observed to be heterogeneously distributed (see supplementary material file in Figure .S1(B)). According to the FESEM images in Figure .2(C&D), the Au NPs contained small pits (indicated by blue arrows, see supplementary file material in Figure .S2(A&B)) and their shape was similar to a smooth/twisted surface, stacked side by side, resembling a cactus plant (indicated by orange arrows, see supplementary file material in Figure .S2(B&C)). The average size of the Au NPs was 33.20 nm. In Figure 3.(E&F), the morphology of the Ag NPs was approximated to that of small spheres arranged around each other with a dimension of 20.02 nm. The FESEM images in Figure S3.(A-C) indicated the presence of spherical structures – formed by the aggregation of small spheres – stacked on top of each other, trapping deep pits between them, resembling wells with a larger area than the pits in the Au and Pt nanocatalyst particles. The TEM images shown in Figure 4.(A-C) reveal the following observations about the internal structure of the nanocatalyst particles: the Pt NPs sheets were rectangular polygons with small spheres in contact with the polygonal boundaries; the Au NPs were heterogeneous spheres with noticeable roughness near them; the Ag NPs were homogeneously spherical throughout their surfaces and had no other structures. The microscopic images (FESEM and TEM) were integrated for all the nanocatalyst particles (Pt NPs, Au NPs and Ag NPs). Complementing the XRD patterns (Figure 1.) and their indications of the purity of the nanocatalyst phases, the EDX spectra (see supplementary material file in Figure S4.(A-C)) and the percentage values of the constituent elements of these nanocatalysts revealed: (i) elemental signals of Pt, Au, and Ag atoms in the fabricated nanocatalyst particles are centered at absorption peaks at around 2.1 keV, 2.3 keV and 2.2 keV, respectively. A homogeneous distribution of each constituent element in the nanocatalyst particle sample was suggested (Figure S4.(A-C)). (ii) The accompanying reports in the inset table for each spectrum (Figure S4.(A-C)) also indicated that the particles of each nanocatalyst exhibited a dominant percentage of Pt in the Pt NPs, Au in the Au NPs, and Ag in the Ag NPs. The EDX spectra also showed other carbon signals due to a very small portion of “EG” remaining stuck on the surface of each nanocatalyst, or believed to be due to the adsorption of carbon dioxide gas on the nanocatalyst surfaces.
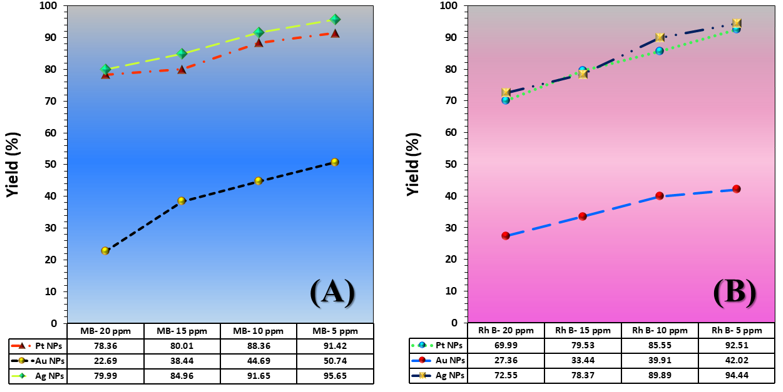
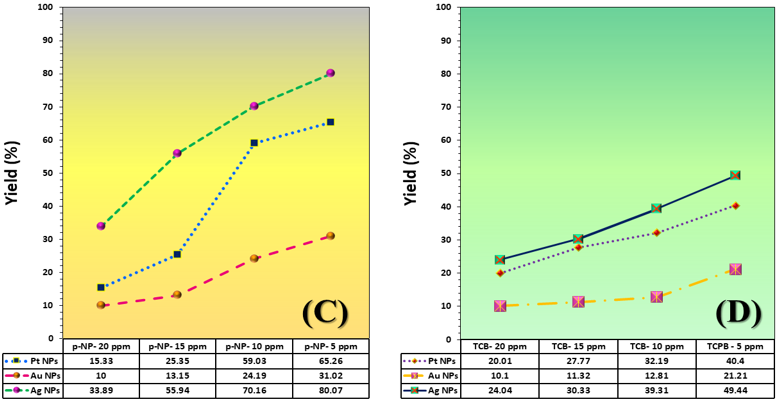
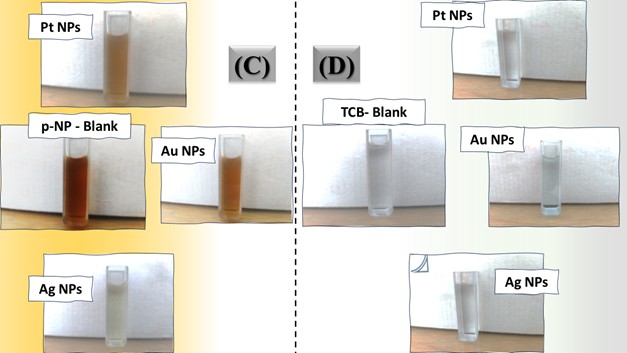
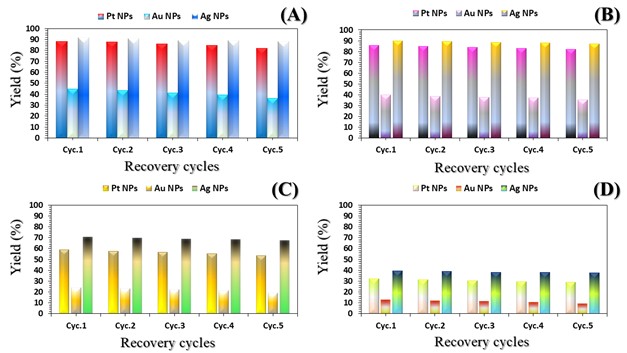
The three catalyst particle structures exhibited diverse nanoscale morphologies and face-centered cubic crystal structures, offering some distinct and promising physiochemical properties. Therefore, these distinct metallic nanocatalyst structures (Pt NPs, Au NPs and Ag NPs) were exploited for practical applications as photocatalysts for four types of contaminants (MB, Rh B, p-NP and TCB) under visible light in the presence of NaBH4. The UV-Vis. Spectra (see supplementary material file in Figures (S5-S8)) showed the characteristic absorption peaks of MB, RhB, p-NP and TCB at 664 nm, 554 nm, 410 nm and 265 nm, respectively, to monitor the photoreduction process for 3h at room temperature, compared to a blank solution of each contaminant at the concentration studied. For comparison, a series of photoreduction tests were also conducted at various concentrations (5, 10, 15 and 20 ppm) under visible light, also with NaBH4 and each nanocatalyst separately. As shown in Figures .5(A-D), the photoreduction tests demonstrated that the nanocatalyst particles differed in performance with each contaminant type and its concentration. The silver-based nanocatalyst particles “Ag NPs” had the highest photoreduction capacity at all contaminant concentrations and for each of the four contaminant types (Figure .4(A-D) & Figure S5.(A-D)). The mixed structure of small spheres and cauliflower buds of Pt NPs demonstrated greater catalytic activity than the large cactus buds against all contaminants (Figure .5(A-D). However, as shown in Figure 6.(A-D), the color of the RhB, MB, p-NP and TCB solutions rapidly changed from colored to colorless. The maximum absorbance of the contaminant solutions decreased significantly over the three-hours reaction time. It was clearly indicated that the photoreduction reaction was completed in three-hours, as shown in Figures.S5-S8 (see supplementary materials file). The slope of the absorbance decrease was significantly greater for the Ag NPs and Pt NPs when comparing the blank solution of each contaminant with the color contaminant solution after photocatalysis and compared to the Au NPs, indicating the excellent catalytic performance of the Ag NPs. It should be noted that the TCB solution was transparent, so that it is difficult to understand the color change that occurred (before and after the photoreduction reaction). However, Ag NPs and Pt NPs not only were more efficient at catalyzing both MB and Rh B than the other two contaminants at low concentrations, but the photoreduction reaction efficiency was also slightly reduced at high concentrations of the preceding contaminants. The colored polyaromatic contaminants (MB and Rh B) were catalyzed rapidly at low concentrations, while the monoaromatic contaminants (p-NP and TCB) were resistant to photocatalysis at both high and low concentrations. Furthermore, the photoreduction reaction of the nanocatalysts fabricated at a concentration of 10 ppm of each contaminant studied over five reuse cycles revealed excellent catalyst reuse rates (Figure .7(A-D)).
DISCUSSION
Currently, developments in the synthesis of nanomaterials based on noble metals, their alloys, corresponding composites, and their excellent ability to reinforce the surfaces of a large number of materials (such as polymers, naturally occurring materials, and oxides), have attracted the attention of environmental researchers. The unlimited chemical and physical properties of these materials greatly facilitate their application in environmental media treatments, chemical reactions, and other processes. To obtain monodisperse nanoparticles of these metals, various protocols were used to form spherical/polygonal/pyramidal/star-shaped particles through solvothermal/hydrothermal reactions, sonication, etc.. The above methods applied specific conditions for each method, and mixtures of organic solvents, especially N, N-dimethyl formaldehyde (DMF), were used to determine the optimal preparation parameters. In general, many of published papers did not pay attention to the green chemistry principles. However, today, a large number of researchers are keen to reduce the potential environmental risks resulting from noble metal nanoparticles preparation processes. Here, green chemistry has emerged in the fabrication process through two important aspects: the use of ultrasonication – as a green fabrication method – and the use of “EG” – as an environmentally friendly solvent -. EG is environmentally safe to the extent required. EG has exceptional properties, including an accelerating agent and morphological regulator, a gentle reducing agent for metal ions, a high boiling point, a medium-polar solvent, a relatively high dielectric coefficient, environmental compatibility, and a good stabilizer. It serves as an important component in the solvothermal method to create a homogeneous structure of metallic nanoparticles. Researchers also studied the mechanism of formation of metallic structures based on EG, and found that this substance acts as an active structure-forming agent and a reducing agent for metal ions. Refuting the formation mechanism is the cornerstone for understanding the crystallographic and morphological changes of the three nanocatalyst particles (Pt NPs, Au NPs and Ag NPs). The related mechanism of the primary particle units was based on (simple/strong) reduction reaction in two successive stages. The reduction reaction in its first stage (shown in Figure 8.) is characterized by the interaction of the metal ions “Mn+” individually (Mn+ = Pt4+, Au3+ and Ag+) with the reducer agent that resulted from the reduction of a portion of EG (23). In the first minutes of heating, the reduction reaction medium was enriched with the glycolaldehyde compound due to oxidation by aerobic oxygen. Its concentration in the solution increased until it reached a certain saturation limit. During the reaction, gradual changes in the glycolaldehyde concentration led to dispersion and an increase in the concentration of the primary nuclei of the related zero-valent metals (Pt(0), Au(0) and Ag(0)). The difference here was the reducing potential of each ion versus the reducing potential of the glycolaldehyde. The redox potentials relative to the hydrogen electrode were as follows: E(0) (PtCl(4-)/Pt) = +0.90 eV, E(0) (AuCl(-1)/Au(0)) = +1.002 eV, E(0) (Ag(+1)/Ag) = +0.791 eV and E(0) (ethylene glycol/glycolaldehyde) = 0.57 V (24). It is noted that the redox potential of the E(0) (ethylene glycol/ glycolaldehyde) is very suitable for a simple reduction reaction of the ions. Each of the formed nuclei had a definite crystal structure. However, because their crystals lack a final surface energy for their crystal facets, they did not assume the final crystalline form. At this stage, they were susceptible to morphological variations and instability. According to the explanations of several researchers (25) (26), the ultra-fine nuclei, each of which served as a precursor for the growth of another nucleus from the related particle. With continuous heating for four hours and constructive collisions between the nuclei, the stability of the ultra-fine particle nuclei was reduced through repeated dissolving, which likely led to recrystallization into larger, more energy-stable crystals (26). Because of reaching very high concentrations and achieving high reductive capacity of glycolaldehyde, prolonged heating with exposure to the largest possible amount of atmospheric oxygen was required. Thus, the second stage of reduction, which is the strong reduction stage under the conditions of the sonication probe method, was ensured by the formation of bubbles in the solution during sonication and their explosion. Both of which were accompanied by high temperatures. Water molecules in the crystalline framework of mineral salts break down, generating free radicals (HO● and H●). These free radicals are naturally very powerful oxidizers, attacking a portion of the EG molecules that have not been converted to glycolaldehyde, forming free radicals of EG. The abundance of these oxidizing free radicals led to further oxidation, producing a medium rich in reducing agents. In parallel with this reaction, the initial nuclei formed composed of the M(0) particles not only augment the activated surface to dissociate the air oxygen and accelerate the initial reaction, but also catalyze the self-reduction of the remained metal ions. Upon completion of the strong reduction stages of the metal ions, a number of intermediate phases emerged that were fruitful in producing the metal particles in their final crystalline forms. The formation of these phases was discussed by considering the functions assigned to each agent, namely: viscosity of the reducing medium, temperature, hydroxyl ions, and hydrazine. Initially, the metal salts dissolve in EG, similar to the dissolution of a metal salt in a weakly polar solvent. The salts quickly transformed into the acidic formulas “HAuO3 ̅2 and HPtO3 ̅” and of both Au and Pt, respectively, similar to what Pan, Karimadom and Fuentes-García reported (27) (28) (29). In this regard, the viscosity of the solution played an important role in the nucleation and reduction stages. The viscosity of the EG solution, having a value of 22 mPa s at 16 °C, decreased with increasing temperature, so that it can orientate the reduction reaction in several ways. First, the decrease in viscosity with increasing temperature enhanced the migration of metal ions in the solution, thereby accelerating and regulating the reduction reaction. In the same context, it also provided crystalline nuclei of M(0) particles at significant concentrations and quantities in the initial stages of the synthesis reaction. Indeed, this was required and important to ensure a favorable initial environment for the final nucleation process. Second, the viscosity of the EG solution implies the presence of two phases (aqueous + organic), which favors the formation of an inverse micelle system (30). In such systems, as discussed in Holade’s paper (31) and consistent with the formation mechanism, the dissolved intermediates of the metals in their ionic state were concentrated within the micelle droplet and surrounded by EG molecules. Then, there is no massive flooding of distorted primary nuclei, as what happened was that a large portion of the ions are protected from random reduction processes and restricted movement. The greatest fruition of this is drawn in the later stages of fabrication – the sonication stage -. After four hours of fabrication, the sonication process of the solution containing the micelle systems (EG/ionic forms of the metal components AuO3 3 ̅&PtO3 2 ̅) began. Free radicals, such as the (HO●, H● and HOCH2CH●OH), penetrated the bicontinuous phase (27) (28) (29) (31). This facilitates the separation of the EG layer – the outer micelle layer – from the ionic constituents of the mineral components – the inner micelle layer -. This caused of the acidic mineral components “HAuO3 and HPtO3” to directly collide with free radicals (HO●, H● and HOCH2CH●OH), generating hydroxyl-based intermediates “[Pt (OH)6]-1, Ag OH and [Au (OH)4]-1“, which is aligns with Kimberly’s proposals (32). According to the findings of Vasilchenko’s paper (33), adjusting the solution medium to become alkaline was valuable in the formation of complex precursors “[Pt (OH)6]-1, Ag OH and [Au (OH)4]-1” of structurally and thermodynamically stable noble metals. Reducing such metallic-hydroxide intermediate phase structures “[Pt (OH)6]-1, [Ag (OH)2] 1 ̅ and [Au (OH)4] 1 ̅” was easily generated stable zero-valent metal structures after their final reduction with hydrazine. The significant function of adjusting the pH value of the solution was also due to the fact that hydrazine’s reducing power increases in alkaline media (34). The good diffusion of micelles creates a steric effect between the particles, forming finely crystallized mineral nuclei for the target particles (Pt NPs, Au NPs and Ag NPs). This was useful for formulating deposits of non-aggregated noble metal particles (Pt NPs, Au NPs and Ag NPs) with a specific crystal structure and spherical or hybrid morphologies. It is inferred from the polyol-based mechanism, as reported in related studies (23) (35) (36), that the noble metal ion reduction and oxygen dissociation reactions proceed without hydroxyl ions, albeit at a very low rate. Regarding reverse micelles, it should be noted that EG undeniably provides a favorable environment for the formation of reverse micelles, similar to the state of reverse micelles, as if a surfactant were present. Due to the pronounced viscosity of EG and its high concentration relative to water droplets (available in mineral salts) at low concentrations, a similar water-in-oil system is formed. EG oxidation products (particularly the glycolaldehyde compound – produced by the oxidation-reduction reaction when the mineral ions are reduced-) play a similar role as surfactants, resulting in the formation of a relatively stable micelle structure (31) (37) (38). There are two experimental observations that led to suggest the formation of such two compounds: (i) Upon examining the pH value of the initial solution formed by the dissolution of the primary salts in EG, it was found to be 1. (ii) A slight change in the color of the resulting initial solutions (in the Au3+/EG solution from yellow to very dark gold, in the Pt4+/EG solution from intense orange to orange-brown, in the Ag+/EG solution from transparent to pale gray) after four hours of continuous stirring at 75°C. Regarding the literature on the possibility of forming such intermediate compounds (“HAuO3 2 ̅ and HPtO3 ̅“), the results of the extensive and clear thermodynamic study in Yuan’s paper on the phrase “gold-chloride-water” prove that acidic and oxidizing conditions provide suitable conditions for the formation of stable acid-oxygen-base complexes of gold (HAuO3 2 ̅). According to the same paper, these complexes are amphoteric in nature and tend to be highly acidic. Therefore, they are easily dissolved in alkaline media and are converted to Au(0). Yuan (39) elaborated in his discussion, particularly when studying the redox (E(0)-pH) curve, that there are a number of intermediate compounds with the formula (HAuO32 ̅, AuO3 3 ̅ and H2AuO3 ̅) that can form as intermediate phases in equilibrium with Au(OH)3 and combine with each other to favor the formation of Au(0) in an alkaline medium. Kurniawan (40) and Malhotra (41) confirmed through electrochemical studies that Au can form relatively stable acidic intermediates and convert to the more structurally stable hydroxide in highly alkaline media. The interaction between the prepared nanocatalysts allowed for a useful correlation between their morphological and crystallographic properties. All three nanocatalysts exhibited a high degree of crystallinity, good crystal orientation, and good crystallite size, and possessed the same crystalline system (FCC system). Repeated recrystallization of small-scale nuclei and their interphase fusion enhanced the metallic bonding in the crystal lattice of the single-cell crystal within the solid zones. The crystallization of irregular and ill-defined nuclei in the soft zones was inhibited during recrystallization. Large-scale orientation of the crystal structures of the nuclei was present in the solid zones. In essence, the formation of the ordered micro-crystalline phase of the target particle nuclei dominates the disordered microcrystalline phases. This is natural, as their formation rate is greater than or equal to the disintegration rate of the disordered microcrystalline phase of the target particle nuclei. The disordered microcrystalline phase disappeared spontaneously with the temperature change between the initial heating stage and the bubble bursting during the sonication stage. This is consistent with the Ostwald ripening principle. On the other hand, the stress alignment of the soft (crystalline and semi-crystalline) zones of the micelles exerted a torque force perpendicular to the strain direction, for growth along the primary crystal growth axis guided by the hard crystalline zones. The nature of these perpendicular forces, which were generated by the crystallized parts of the soft zones, reduces the deformation of the formed crystals. Furthermore, the excessive elongation of the EG chains within the micelle structure caused the solid zones of the small-scale nuclei to reorient and restructure along the crystal axis. This effect increased with the distribution and dissipation of stresses, encouraging the formation of a uniform crystalline system of nanocatalyst particles. Undeniably, the slightly crystalline micelles acted as large-strain dampers, meaning they reduced crystal distortion. This indicates that the solid zone reflections observed in the diffraction patterns, represented by the (1 1 1) peak, were due to the good alignment of crystals in these zones, which were held together by strong metallic bonds (42). This results in good crystallinity indices (degree of crystallinity and orientation). The results (Table 1) shows that the platinum-based catalyst particles have the lowest crystallinity indices compared to the other two catalyst particles (Au NPs and Ag NPs). This was attributed to the presence of two morphologies (spherical and sheet) (21). Sheets were rarely observed in Pt NPs (Figure 3(A&B)), but a logical explanation for this is that their primary nuclei grew in all directions and that platinum ions exhibited poor reductive capacity in EG (34). The polyol method, on the other hand, typically favors gold’s ion reduction reaction and the formation of more homogeneous and uniform morphologies. Silver is most responsive to the reduction of its ions in EG to Ag(0). The last observation worth noting is that the large crystallite size values of the Ag-nanocatalyst and Au-nanocatalyst explain the reason for the sharpness of the reflection peak, unlike the Pt-nanocatalyst. The {1 1 1} facet is the lowest-energy facet, and there is order in the formed polycrystals and low surface roughness, as in the case of the Ag-nanocatalyst. The {1 1 1} facet in Pt NPs is lower in energy than the {1 1 0} facet, resulting in a predominantly spherical morphology, which requires a more regular crystal structure than gold. In other words, the effect of both crystalline facets can be seen in Pt- and Au-based nanoparticles. In essence, the formation of the fine crystalline phase of the target particle nuclei dominates the amorphous microphases. This is natural, as the rate of their formation is greater than or equal to the rate of disintegration of the disordered crystalline phase of the target particle nucleus. The final phase disappears spontaneously with the temperature change between the initial heating stage and the bubble bursting during the sonication stage. This is consistent with the principle of estuarine maturation. As seen in Figure 5, the catalytic performance of the three particles can be ordered according to the type of nanocatalyst particle, the type of contaminant, and its concentration as follows:
For particle type: Ag NPs > Pt NPs > Au NPs
For contaminant type: MB > Rh B > p-NP > TCB
For contaminant concentration: 5 ppm > 10 ppm > 15 ppm > 20 ppm
It is perhaps important to establish a link between the laboratory photoreduction reaction results in the presence of NaBH4 and the structure of the three catalyst particles. The first link (crystal structure and photocatalysis) is that since crystals have a {1 1 1} facet, without mixtures with {1 1 0} facet, at the lowest possible energy, the catalytic sites can be controlled and encouraged to complete the catalytic reaction in the best possible way. Mixture of {1 1 1} and {1 1 0} crystal facets, such as Pt NPs and Au NPs, pose energetic barriers to catalysis because they lack the activation energy required to complete the reaction. Crystals originating from finer nucleation centers exhibit better reactivity in photophysical reactions, as in Ag-nanocatalysts first, followed by Pt-nanocatalysts. Such well-crystalized centers have the opportunity to interact with light and create electronic transitions and form free radicals (●OH and ●O2̄ ) that accelerate the catalytic reaction. Au-nanocatalyst, with a crystallinity between that of Pt-nanocatalyst and Ag-nanocatalyst, did not have the advantage of sufficiently interacting with light and producing free radicals (●OH and ●O2̄ ). The higher crystallinity degree of Ag NPs (60.29%) and lower crystallinity degree of Pt NPs (46.64%) and Au NPs (50.07%) means that the surface area of Ag NPs was increased, securing sites along their entire surface and utilizing them as sites for free radical oxidation. However, the effect of crystal crystallite sizes stems from their influence on the electronic state (particularly the conduction and valence bands). Scientific observations suggest that this generates energetically active intermediates and a huge number of photoactive agents, which further accelerates the photoreduction reaction (36) (43). The second link is (morphology and photocatalysis). Typically, the entire photoreduction reaction depends on the morphology of the particles or the prepared nanostructure. Ag-nanocatalyst particles, which yielded the best photoreduction performance, had a uniform and homogeneous spherical surface morphology. Spherical morphology, a type of zero-dimensional morphology, adsorbed electrons at the same rate in all dimensions (x, y, z) (45). As shown in the FESEM (Figure 3(E&F)) and TEM images (Figure 4(C)), the pits trapped between the spherical particles were small and deep, creating a morphologically impermeable internal surface (21). When light penetrates the surface of the impermeable structure, electrons – generated by the interaction of the Ag-nanocatalyst particle’s surface with visible light radiation – fall into the pits and become trapped (21). This accelerates the collision rate with the Ag NPs and creates a stream of the photoactive agents. The Ag spheres increase the adsorption order of BH4̄ on its surface and encourage rapid movement of the liberated hydrogen across the surface of this nanocatalyst. The last two observations highlight how morphological features, through their synergistic effects on the dispersion of photoactive species and facilitating hydrogen transfer, can contribute to improved photoreduction efficiency. However, the presence of a spherical structure in the Pt-nanocatalyst particles had an impact on the improved catalytic performance. While the presence of a lamellar structure did provide good catalytic performance for Pt-nanocatalyst particles, its catalytic performance differed slightly from that of Ag-nanocatalyst particles. The reason is the homogeneity of the surface morphology of the Ag-nanocatalyst. It appears that structural heterogeneity of the Pt-nanocatalyst reduced its catalytic performance. Regarding the sheet’s structure, the following observation can be conclusively concluded: the corner sites in the short, thin, polygonal sheets are more highly occupied than others, and exhibit very good selectivity for the adsorption of hydrogen liberated from BH4̄. Pt-nanocatalyst particles could have demonstrated better catalytic performance if they had a larger number of sheet sites, resulting in a higher edge-to-corner ratio (43), as reported in Zhou’s research. Similarly, Au-nanocatalyst particles with pits of different sizes (large and small) and an agglomerated polygonal structure deteriorated the catalytic performance. The catalytic performance of nanoporous noble metal catalysts (Pt/Au-nanocatalysts) is related to the compressive strain factor. Two types of pits can be detected in such structures (primary pits and secondary pits), according to Malekian (46), intertwined within the same Au-nanocatalyst morphology. The existing agglomeration and pits of different sizes can induce differential compressive strain and deformation in these pits. The deformation is large in large pit structures and small in small pit structures (46). Because large pits are more resilient to compression, the creation of large compressions by the agglomerates significantly reduces the pit size and, in turn, affects small pit to almost the same extent. It should also be noted that if the agglomerated structure itself is porous, its effect is different and separate from the compressive strain in noble metal structures (46). This topic will not be discussed in the current study because the Au-nanocatalyst agglomerated structure is not porous. Therefore, it is very likely that the compressive strain was not large and the adsorption energy was insufficient, which reduced the adsorption of liberated hydrogen and the generation of photoactive agents. This resulted in a reduction in the catalytic performance of the Au-nanocatalyst surface. Returning to the discussion of contaminant type, the two contaminants (MB and Rh B) were the easiest and fastest to photocatalyze compared to the two petroleum contaminants (p-NP and TCB). The colored polyaromatic heterocyclic contaminants (MB and Rh B), due to their π-electrons and (HOMO-LUMO) system, are able to transition to an excited state (MB* and Rh B*) upon collision with photons of light. When the excited states of MB* and Rh B* return to their ground states, a certain amount of energy is released. This energy is complemented by the energy released by photon collisions with individual nanocatalyst particles. Whereas, the two petroleum contaminants consist of a single homologue aromatic ring, making electronic excitation difficult. However, the presence of hydroxyl groups in p-NP compared to chlorine groups in TCB makes the phenol ring more active for the photoreduction reaction than in TCB. Finally, increasing the concentration of any contaminant caused a downward slope for the photoreduction reaction. A thicker and thicker layer of contaminant surrounded the nanocatalyst surface as its concentration increased. This increased layer reduced the penetration of light to conduct electronic excitation and the transfer of hydrogen liberated from the BH4¯ to the nanocatalyst layers (either Pt-nanocatalyst or Au-nanocatalyst), suggesting a lower photoreduction rate. Considering the above reasonings and the mechanism proposed by Shafiq (35), the proposed photoreduction mechanism was attributed to two complementary pathways: generation/transfer of photoactive agents, and hydrogen donor/movement. Initially, two components (contaminant molecules and BH4 ions) were adsorbed simultaneously. Here, the adsorption occurred due to a charge difference, as the components (MB, Rh B and BH4¯) dissolved in the aqueous medium are negatively charged, while the catalyst particles are positively charged. Furthermore, the characteristics of each component involved in the photocatalysis (NaBH4, contaminant, nanocatalyst) were, respectively: nucleophilic, electrophilic, surface-organized for hydrogen movement, and photoactive-generating. The catalyst surface interacted with photons to generate electron excitation from the ground band to the valence band. At the same time, the electrophile molecules were excited, generating a stream of electrons and holes. These can react with water molecules, destroying them and generating free radicals (●OH and ●H). The nanocatalyst surface was activated to regulate the presence and migration of hydrogen and deliver photoactive agents to the catalytic reaction medium. This system reflected the behavior of the nanocatalyst enhanced with NaBH4 against the four contaminants (23) (35) (36) (47) (48) (49) (50).
CONCLUSIONS AND RECOMMENDATIONS
In conclusion, a simple, green strategy is reported, detailing most of the steps involved in the fabrication of a set of three noble metal nanocatalysts for Ag NPs, Au NPs and Pt NPs. The nanocatalyst backbones were well-structured, pure, and shared diverse nano-spherical shapes, while the Au NPs and Pt NPs nanospheres exhibited polygonal, agglomerated morphologies and sheet morphologies. This strategy provided an efficient way for fabricating nanoparticles of the three noble metals, creating a strong bond between their structural and crystalline characteristics. Due to this bond, a deeper study was conducted on the catalytic reduction performance of these metal nanoparticles in the reduction reaction of four toxic contaminants (MB, Rh B, p-NP and TCB) at varying concentrations, under visible light irradiation and using NaBH4. The results demonstrated outstanding catalytic reduction behavior, excellent stability, and reusability of each nanocatalyst. After extensive discussion, this work revealed the design of the Ag-nanocatalyst for organic pollutant catalytic applications through rational structural integration of its nanoparticles. The Pt-nanocatalyst came in second place, followed by the Au-nanocatalyst. It is believed that the presented concepts should be applied to a wide range of applications by studying the following proposals: constructing other hybrid materials from these metallic particles, functionally modifying their surfaces with natural polymeric substrates, and exploring other green and sustainable methods for fabricating them with new structural specifications. It is also suggested to complement these studies by conducting analyses such as GC-MS, HPLC, and NMR, calculating environmental indicators to assess the toxicity of the resulting compounds and those released into the environment, such as POD, COD, and TOC, and estimating indicators specific to catalytic reactions, such as TOF and TON. It is also suggested to enhancethe catalytic functions of the fabricated nanoparticles so that they can be applied in the field of photodegradation.
References :[1] Al-Tohamy R, Ali SS, Li F, Okasha KM, Mahmoud YA, Elsamahy T, Jiao H, Fu Y, Sun J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicology and environmental safety. 2022 Feb 1;231:113160.
[2] Boulkhessaim S, Gacem A, Khan SH, Amari A, Yadav VK, Harharah HN, Elkhaleefa AM, Yadav KK, Rather SU, Ahn HJ, Jeon BH. Emerging trends in the remediation of persistent organic pollutants using nanomaterials and related processes: A review. Nanomaterials. 2022 Jun 22;12(13):2148.
[3] Karbalaee Hosseini A, Tadjarodi A. Luminescent Cd coordination polymer based on thiazole as a dual-responsive chemosensor for 4-nitroaniline and CrO42− in water. Scientific Reports. 2023 Jan 6;13(1):269.
[4] Alharbi OM, Khattab RA, Ali I. Health and environmental effects of persistent organic pollutants. Journal of Molecular Liquids. 2018 Aug 1;263:442-53.
[5] Purkait, M. K., DasGupta, S., De, S. (2005). Adsorption of eosin dye on activated carbon and its surfactant based desorption. Journal of environmental management, 76(2), 135-142.
[6] González-Mille DJ, Ilizaliturri-Hernández CA, Espinosa-Reyes G, Costilla-Salazar R, Díaz-Barriga F, Ize-Lema I, Mejía-Saavedra J. Exposure to persistent organic pollutants (POPs) and DNA damage as an indicator of environmental stress in fish of different feeding habits of Coatzacoalcos, Veracruz, Mexico. Ecotoxicology. 2010 Oct;19:1238-48.
[7] Abu-Nada A, Abdala A, McKay G. Removal of phenols and dyes from aqueous solutions using graphene and graphene composite adsorption: a review. Journal of Environmental Chemical Engineering. 2021 Oct 1;9(5):105858.
[8] Gupta A, Thakur IS. Treatment of Organic Recalcitrant Contaminants in. Biological wastewater treatment and resource recovery. 2017 Mar 29:1.
[9] Tee GT, Gok XY, Yong WF. Adsorption of pollutants in wastewater via biosorbents, nanoparticles and magnetic biosorbents: A review. Environmental Research. 2022 Sep 1;212:113248.
[10] Hosseini AK, Tadjarodi A. Sonochemical synthesis of nanoparticles of Cd metal organic framework based on thiazole ligand as a new precursor for fabrication of cadmium sulfate nanoparticles. Materials Letters. 2022 Sep 1;322:132481.
[11] Najjar R, Nematdoust S. A resistive-type humidity sensor based on polypyrrole and ZnO nanoparticles: hybrid polymers vis-a-vis nanocomposites. RSC advances. 2016;6(113):112129-39.
[12] Shen R, Ren D, Ding Y, Guan Y, Ng YH, Zhang P, Li X. Nanostructured CdS for efficient photocatalytic H2 evolution: A review. Science China Materials. 2020 Nov;63(11):2153-88.
[13] Farooqi ZH, Begum R, Naseem K, Wu W, Irfan A. Zero valent iron nanoparticles as sustainable nanocatalysts for reduction reactions. Catalysis Reviews. 2022 Apr 3;64(2):286-355.
[14] Wang R, Ma X, Liu T, Li Y, Song L, Tjong SC, Cao L, Wang W, Yu Q, Wang Z. Degradation aspects of endocrine disrupting chemicals: A review on photocatalytic processes and photocatalysts. Applied Catalysis A: General. 2020 May 5;597:117547.
[15] Loza K, Heggen M, Epple M. Synthesis, structure, properties, and applications of bimetallic nanoparticles of noble metals. Advanced functional materials. 2020 May;30(21):1909260.
[16] Liu L, Gu X, Cao Y, Yao X, Zhang L, Tang C, Gao F, Dong L. Crystal-plane effects on the catalytic properties of Au/TiO2. Acs Catalysis. 2013 Dec 6;3(12):2768-75.
[17] Kara GK, Tadjarodi A. Designing a novel method for metallization polyacrylonitrile nanofiber surface by noble metallic nanoparticles: A study of synergistic relation between structural features and the mechanical/wetting properties. Express Polymer Letters. 2022 May 1;16(5).
[18] Kara GK, Tadjarodi A, Kehtari M. Designing a novel 3D nanofibrous scaffold based on nanoalloy AuAg NPs (AuAg@ PAN NFs) for osteogenic differentiation of human adipose derived mesenchymal stem cells (hADMSCs). European Polymer Journal. 2022 Mar 15;167:111073.
[19] Beni AA, Jabbari H. Nanomaterials for environmental applications. Results in Engineering. 2022 Sep 1;15:100467.
[20] Zheng Y, Zheng L, Zhan Y, Lin X, Zheng Q, Wei K. Ag/ZnO heterostructure nanocrystals: synthesis, characterization, and photocatalysis. Inorganic chemistry. 2007 Aug 20;46(17):6980-6.
[21] Kara GK, Moshari M, Rabbani M, Rahimi R. A novel and green heterogeneous photocatalytic system (Ca0. 01Fe2.99O4/CaTiO3 nanocomposite): Protocol synthesis, characterization, and study of photo-decoloration activity. Materials Chemistry and Physics. 2021 Feb 1;259:124062.
[22] Kou Y, Guo Y, Liang L, Li X, Wang Y, Su P, Yan CH, Tang Y. Electrostatic Self‐Assembly of Ag‐NPs Mediated by Eu3+ Complexes for Physically Unclonable Function Labels. Aggregate. 2025 Mar;6(3):e701.
[23] Rajendiran R, Seelam PK, Patchaiyappan A, Balla P, Shankar H, Ravi B, Perupogu V, Lassi U. Morphologically tailored facet dependent silver nanoparticles supported α-Al2O3 catalysts for chemoselective reduction of aromatic nitro compounds. Chemical Engineering Journal. 2023 Jan 1;451:138507.
[24] dos Santos Pereira F, de Santana VF, Silva AC, Tofanello A, Romano PN, de Almeida JM, Rodrigues TS, de Araujo Rodrigues I, de Lima RB, Garcia MA. Electrooxidation of ethylene glycol using Ag-Pt nanotubes supported on silica: Correlating the unexpected O-vacancies creation with catalytic performance. Catalysis Today. 2024 Nov 1;441:114914.
[25] Feng Y, Ma X, Wang S, Wang X, Feng J, Wen J, Tian Y. The role of oxygen in the growth of silver nanowires using the polyol method. Materials Letters. 2024 Dec 1;376:137290.
[26] Thomas N, Sharma N, Swaminathan P. Optimizing silver nanowire dimensions by the modification of polyol synthesis for the fabrication of transparent conducting films. Nanotechnology. 2023 Nov 16;35(5):055602.
[27] Pan L, Zhang X, He S, Zhang G, Xu W. Eco-Friendly and Efficient Extraction of AU from Waste Mobile Phones Using a Sodium Persulfate-Potassium Iodide System. Available at SSRN 4013267. 2022.
[28] Fuentes-García JA, Santoyo-Salzar J, Rangel-Cortes E, Goya GF, Cardozo-Mata V, Pescador-Rojas JA. Effect of ultrasonic irradiation power on sonochemical synthesis of gold nanoparticles. Ultrasonics Sonochemistry. 2021 Jan 1;70:105274.
[29] Karimadom BR, Kornweitz H. Mechanism of producing metallic nanoparticles, with an emphasis on silver and gold nanoparticles, using bottom-up methods. Molecules. 2021 May 17;26(10):2968.
[30] Sharma K, Kaushal S, Jain A, Sami MH, Kumar S, Tariq H, Bano K, Aggarwal S, Kumar R, Singh PP. A comprehensive review on biogenic synthesis of bimetallic nanoparticles and their application as catalytic reduction of 4-nitrophenol. Chemical Papers. 2024 Apr;78(5):2757-82.
[31] Holade Y, Sahin NE, Servat K, Napporn TW, Kokoh KB. Recent advances in carbon supported metal nanoparticles preparation for oxygen reduction reaction in low temperature fuel cells. Catalysts. 2015 Mar 6;5(1):310-48.
[32] Kimberly TQ, Frasch MH, Kauzlarich SM. Colloidal synthesis of two-dimensional nanocrystals by the polyol route. Dalton Transactions. 2024;53(32):13280-97.
[33] Vasilchenko D, Berdyugin S, Komarov V, Sheven D, Kolesov B, Filatov E, Tkachev S. Hydrolysis of [PtCl6]2–in concentrated NaOH solutions. Inorganic Chemistry. 2022 Apr 5;61(15):5926-42.
[34] Vilardi G, Verdone N, Bubbico R. Combined production of metallic-iron nanoparticles: exergy and energy analysis of two alternative processes using Hydrazine and NaBH4 as reducing agents. Journal of the Taiwan Institute of Chemical Engineers. 2021 Jan 1;118:97-111.
[35] Shafiq A, Deshmukh AR, AbouAitah K, Kim BS. Green synthesis of controlled shape silver nanostructures and their peroxidase, catalytic degradation, and antibacterial activity. Journal of Functional Biomaterials. 2023 Jun 18;14(6):325.
[36] Rahman S, Al-Harbi FF, Ajmal M, Naseem A, Farooqi ZH, Siddiq M. Engineering of micron-sized spherical anionic microgel fabricated with silver nanoparticles with antimicrobial and catalytic potential. Journal of Materials Science. 2022 Mar;57(12):6763-79.
[37] Ruiz-Sánchez A, Lapidus GT. Electrochemical and leaching studies to better understand the role of ethylene glycol in the oxidative acid dissolution of chalcopyrite. Electrochimica Acta. 2022 Jun 20;418:140343.
[38] Agazzi FM, Falcone RD, Silber JJ, Correa NM. Non-aqueous reverse micelles created with a cationic surfactant: Encapsulating ethylene glycol in BHDC/non-polar solvent blends. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2016 Nov 20;509:467-73.
[39] Yuan X, Tang DW, Zou T, Xu CW, Qiu Y. Combined leaching of Carlin-type gold deposit in Guizhou by potassium chlorate and bleaching powder. Materials Research Express. 2022 Dec 9;9(12):126506.
[40] Kurniawan A, Kurniawan F, Gunawan F, Chou SH, Wang MJ. Disposable electrochemical sensor based on copper-electrodeposited screen-printed gold electrode and its application in sensing L-Cysteine. Electrochimica acta. 2019 Jan 10;293:318-27.
[41] Malhotra S, Tang Y, Varshney PK. Amperometric Enzyme-Free Glucose Sensor Based on Electrodeposition of Au Particles on Polyaniline Film Modified Pt Electrode. Acta Chimica Slovenica. 2018 Sep 1;65(3).
[42] Waletzko RS, Korley LT, Pate BD, Thomas EL, Hammond PT. Role of increased crystallinity in deformation-induced structure of segmented thermoplastic polyurethane elastomers with PEO and PEO− PPO− PEO soft segments and HDI hard segments. Macromolecules. 2009 Mar 24;42(6):2041-53.
[43] Wang X, S.L, Su R, Wendt S, Hald P, Mamakhel A, Yang C, Huang Y, Iversen BB, Besenbacher F. The influence of crystallite size and crystallinity of anatase nanoparticles on the photo-degradation of phenol. Journal of catalysis. 2014 Feb 1;310:100-8.
[44] Roy E, Patra S, Saha S, Kumar D, Madhuri R, Sharma PK. Shape effect on the fabrication of imprinted nanoparticles: Comparison between spherical-, rod-, hexagonal-, and flower-shaped nanoparticles. Chemical Engineering Journal. 2017 Aug 1;321:195-206.
[45] Chu S, Zhou W, Zhang C, Zheng Y, Liu Y, Liu Y. Relationship between the structure and catalytic performance of MoS 2 with different surfactant-assisted syntheses in the hydrodesulfurization reaction of 4,6-DMDBT. Rsc Advances. 2020;10(13):7600-8.
[46] Malekian A, Salari S, Stumper J, Djilali N, Bahrami M. Effect of compression on pore size distribution and porosity of PEM fuel cell catalyst layers. international journal of hydrogen energy. 2019 Aug 30;44(41):23396-405.
[47] Fouladi-Fard R, Aali R, Mohammadi-Aghdam S, Mortazavi-derazkola S. The surface modification of spherical ZnO with Ag nanoparticles: A novel agent, biogenic synthesis, catalytic and antibacterial activities. Arabian Journal of Chemistry. 2022 Mar 1;15(3):103658.
[48] Alula MT, Aragaw BA, Modukanele ST, Yang J. Enhanced catalytic activity of silver nanoparticles loaded into Fe3O4 nanoparticles towards reduction of 4-nitrophenol, degradation of organic dyes and oxidation of o-phenylenediamine. Inorganic Chemistry Communications. 2021 May 1;127:108504.
[49] Liu L, Corma A. Structural transformations of solid electrocatalysts and photocatalysts. Nature reviews chemistry. 2021 Apr;5(4):256-76.
[50] Mohammadi R, Alamgholiloo H, Gholipour B, Rostamnia S, Khaksar S, Farajzadeh M, Shokouhimehr M. Visible-light-driven photocatalytic activity of ZnO/g-C3N4 heterojunction for the green synthesis of biologically interest small molecules of thiazolidinones. Journal of Photochemistry and Photobiology A: Chemistry. 2020 Nov 1;402:112786.
Competing Interests :The authors declare there is no competing of interest.
Fund: No fund.
Author contributions: Ghaffar Kh. Kara Investigation, Supervision, Formal analysis, Conceptualization, Methodology, Funding Acquisition, Visualization, Validation, Writing – original draft, Resources. Muna Qareh Writing, Reviewing, Methodology, Validation. Maya Zanouba Formal analysis, Writing. Sedra Alsyed Editing, Writing – original draft, Visualization.
Data and materials availability: All data and materials used in the analysis are available in the article and supplementary materials, and further information could be provided upon contact with corresponding author.
Supplementary Materials: All data and materials used in the analysis are available in the article and supplementary materials, and further information could be provided upon contact with corresponding author.
(ISSN - Online)
2959-8591