Association of ACE2 Rs2074192 and Rs233574 and AGT Rs699 Gene Polymorphisms with Obesity and Disease Severity In A Cohort Of COVID-19 Affected Syrians
2024-07-18 | volume 2 Issue 2 - Volume 2 | Research Articles | Majd AljamaliAbstract
Since the year 2020, accumulating data have identified numerous factors playing cardinal role in inter-individual variations for COVID-19 morbidity including age, obesity, hypertension, comorbidities, in addition to genetic susceptibility. Among the key genes that might contribute to variable clinical outcomes in patients are those involved in the renin-angiotensin-aldosterone system (RAAS); namely angiotensin I and II (ACE, ACE2) and angiotensinogen (AGT). In this study, the allele and genotype frequencies of three single nucleotide polymorphisms (SNPs); ACE2 rs2074192 and rs233574 in the ACE2 gene, and rs699 in the AGT gene were recorded in a cohort of Syrian subjects previously suffered from COVID-19 disease with variable symptoms and comorbidities. Fifty-four subjects previously infected by COVID-19 disease were interviewed and classified based on three categories; according to blood pressure levels [normotensive (NT) or hypertensive (HT)], obesity [lean or overweight and obese (OW/OB)], and COVID-19 conditions [mild or moderate and severe (MOD/SV)]. Genotyping of the three SNPs was performed on DNA extracted from blood samples taken from the interviewed subjects followed by electrophoresis and DNA sequencing. We found higher allele frequencies of rs2074192 C allele, rs233574 T allele, and rs699 C allele in OW/OB females (79.2%, 45.2%, and 62.5%, respectively) compared to lean females (33.3%), p values (0.066, 0.079, and 0.043, respectively). Furthermore, the recorded association of higher allele frequencies of the three SNPs with either obesity or COVID-19 severity suggested the C allele for rs2074192, the T allele for ACE2 rs233574, and the C allele of AGT rs699 as risk factors for obesity only in female subjects and COVID-19 severity only in male subjects. Taken together, we proposed two rs2074192-rs233574-rs699 haplotypes, CC-TT-CC in females and C-T-CC in males, as risk haplotypes for COVID-19 severity in our Syrian cohort. Further studies with a larger number of COVID-19 affected Syrian subjects should be performed to confirm our preliminary results.
Keywords : Gene polymorphism, ACE2, AGT, rs2074192, rs233574, rs699, Obesity, COVID-19 severity.
INTRODUCTION
The COVID-19 pandemic in recent years has placed a huge burden on health care systems worldwide, as well as intriguing health personnel and scientists who stood obscured with its wide-ranged clinical phenotype in patients, i.e. from regular influenza symptoms to acute respiratory distress syndrome (ARDS), and possible death [1,2]. Moreover, the infection with corona virus was accompanied by severe inflammatory and immune responses, represented by a cytokine storm, often leading to severe lung damage and requiring patient admission to intensive care units (ICUs) and mechanical ventilation at local hospitals [3,4]. The main cellular receptor for corona virus, which facilitates viral entry into cells, is angiotensin converting enzyme 2 (ACE2), a cellular receptor and a main component of the renin-angiotensin-aldosterone system (RAAS), with enzymatic activity that hydrolyzes angiotensin-II (Ang-II) into Ang-1-7 [5,6]. A second important player in the RAAS system is angiotensinogen (AGT), a peptide hormone encoded by the AGT gene, which is cleaved by the renin enzyme to produce angiotensin I (Ang-I). The enzyme angiotensin (ACE or ACE1) then converts Ang-I to Ang-II [7,8]. Ang-II binds to the AT1 receptor, mainly associated with vasoconstriction, fibrosis, inflammation and thrombosis. On the contrary, Ang-1-7, resulting from ACE2 hydrolysis of Ang-II, binds to the AT2 receptor, resulting in dilation of blood vessels and reduction of fibrosis, inflammation, and thrombosis. Thus, ACE2 produces a protective response in the lung, while high ACE activities are associated with lung and cardiovascular diseases by increasing the activity of the Ang-II/AT1R axis [5,9]. Since the beginning of the COVID-19 pandemic, a plethora of scientific reports have highlighted the importance of age and several comorbidities, including obesity and hypertension, in worsening the clinical phenotype in COVID-19 patients, linking the disruption of homeostasis in the RAAS, the immune responses, and other physiological systems to severe clinical outcomes [8,10]. Many other reports have revealed associations between COVID-19 severity and genetic variants in genes associated with the RAAS including ACE, AGT and ACE2 [11-15]. Among the key player genes that received much attention after the pandemic was the ACE2 gene. On one hand, many genetic polymorphisms were identified in ACE2 that could change the binding affinity of the corona virus spike protein, hence, increasing or decreasing its entry into target cells [12]. On the other hand, and because of ACE2 major involvement in RAAS regulation, several ACE2 gene polymorphisms were identified and linked to predisposing COVID-19 patients to comorbidities that could worsen their clinical path [16,17]. One intronic single nucleotide polymorphism (SNP) in ACE2, rs2074192, has been linked to hypertension and severity of COVID-19 [18-21]. Another intronic SNP in ACE2 gene, rs233574, has been linked to stability of ACE2 mRNA and protein [22,23]. Moreover, few reports identified an exonic SNP in the AGT gene, rs699 or M268T, which replaces methionine residue with threonine in the angiotensinogen peptide and was showed to be associated with increased susceptibility to COVID-19 [7,24]. Nevertheless, it appeared that the effects of the three aforementioned SNPs in ACE2 and AGT genes on COVID-19 severity were correlated with the studied population ethnicities, as data linked to the involvement of these SNPs in COVID-19 morbidities and co-morbidities often came contradictory, taking into consideration the varied allelic frequencies among different ethnic groups. Hence, in this report, we studied the association of the two SNPs in the ACE2 gene, rs2074192 and rs233574, in addition to the AGT rs699 SNP, with COVID-19 disease severity in a cohort of Syrian patients whose corona virus infection was previously confirmed by standard methods. We reported the allele, genotype and haplotype frequencies of the three polymorphisms in both sexes after categorizing the patients according to obesity, hypertension and COVID-19 severity.
MATERIALS AND METHODS
Subjects and Samples
Our retrospective cohort study included 54, 24 females and 30 males, non-related participants with previously confirmed COVID-19 positive disease, by PCR positive tests (22 cases) or positive chest X-ray in addition to clinical symptoms (22 cases) or positive Corona IgG (10 cases). The Study cases were classified based on 3 categories: blood pressure into (39 normotensive NT) and (15 hypertensive HT); obesity, reflected by body mass index (BMI), into (20 lean, BMI<25) and (34 overweight OW, 25<BMI<30 and obese OB, BMI>30); and finally COVID-19 status into (21 mild cases, mild symptoms) and (33 moderate cases including 17 with intense symptoms but oxygenation level >94, and 13 severe cases with intense symptoms, and 3 cases with life-threatening conditions and oxygenation level <94). Our study was approved by both the Bioethics Committee at the Faculty of Pharmacy – Damascus University, and the National Committee for Ethics of Scientific Research and Novel Technologies (CONEST), at the Higher Commission for Scientific Research. Informed consents were obtained from all subjects prior to their enrollment. Three milliliters of peripheral blood were collected on Ethylene Diamine Tetra-acetic Acid (EDTA) and stored at -20 °C.
DNA Isolation and Amplification
Molecular work and genotyping processes were performed at the pharmaceutical biotechnology and immunology laboratories- National Commission for Biotechnology, Damascus, except for DNA sequencing. Genomic DNA was isolated from blood samples using Qiagen blood DNA extraction kit (Qiagen, USA) according to the manufacturer’s protocol. DNA concentration and purity were assessed using Nano drop (Maestrogen®, Taiwan). The extracted DNA quality was verified by horizontal 1.5% agarose gel electrophoresis, followed by gel staining with ethidium bromide staining (Promega®, USA), and gel documentation (Cleaver, UK). We performed polymerase chain reaction (PCR) amplification using two sets of specific primer pairs. For both ACE2 rs2074192 and rs233574, we used forward primer 5` CAGCAAAGGGGACACTTAGACA 3` and reverse primer 5` AGCCATTTCCCATCCCAGTG 3`, with a theoretical expected amplicon size of 707 base pair (bp). For AGT rs699, we used forward primer 5` GTGGTCACCAGGTATGTCCG 3` and reverse primer 5` TATACCCCTGTGGTCC TCCC 3`, with an expected amplicon size of 291 base pair (bp). All primers were manufactured by (Eurofins, Belgium), and were designed using Primer 3 software tool and checked prior to ordering using the MFE primer bioinformatics tool (https://mfeprimer3.-igenetech.com/spec) to evade any non-specific binding to genomic DNA. PCR was performed according to standard methods using thermal cycler (SENSEQUEST®, Germany) and 2X Master Mix (Genedirex®, Taiwan). The PCR reaction mixture contained 20 ng of genomic DNA and 0.25 µm/l of each primer. PCR amplification was performed according to the following protocol including: initial denaturation for 5 min at 95° C, 35 cycles of (30 sec at 94° C, 30 sec at 57° C, and 1 min at 72° C), and a final 10 min at 72° C for final extension. PCR amplicons were analyzed by agarose gel electrophoresis, using an electrophoresis apparatus from (Cleaver, UK) and a 100 bp DNA size marker from (Genedirex®, Taiwan). DNA Sequencing was performed at (Macrogen, South Korea) according to standard protocols.
Bioinformatics & Statistical Analyses
Sequencing chromatograms were analyzed using a bioinformatics tool (Geneious® software, USA). Genotype frequencies of sample alleles were estimated by gene counting method. Statistical analyses were performed using chi-square calculator https://www.socscistatistics.com/tests/ to compare the ratios of allele and genotype frequencies, respectively, with a p-value <0.05 as significant. Finally, EXCEL T test calculator was used to compare means of age and BMI among study cases, with a p-value <0.05 for statistical significance.
RESULTS
Subjects
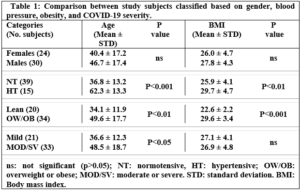
No significant differences were recorded concerning age and body mass index (BMI) of females and males, (Table 1). However, when subjects were divided according to blood pressure status and classified as NT and HT groups, both age and BMI were significantly higher in HT (40 yrs. and 36.8 kg/m2) compared to NT (46.7 yrs. 29.7 kg/m2), respectively, (Table 1). Additionally, overweight (OW) and obese (OB) subjects showed a significantly higher mean age (49.6 yrs.) compared to lean subjects (age 34.4 yrs.). Finally, subjects with moderate or severe COVID-19 conditions (MOD/SV) were significantly older than those with mild conditions (48.5 versus 36.6 yrs., respectively), while no significant difference appeared in BMI between MOD/SV and mild conditions.
Genotyping
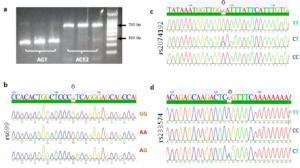
Amplification of the ACE2 and AGT gene sequences was successful, with amplicons appeared with expected molecular weights (MW), ~ 700 bp for ACE2 and between 200 and 300 bp for AGT, (Fig. 1 a). For all three SNPs, homozygotes and heterozygotes were easily identified by reading the sequencing chromatograms; (GG/CC, AA/TT, or AG/TC) for rs699, (CC, CT, or TT) for rs2074192, and (TT, CC, or CT) for rs233574, (Fig. 1, b-d).
Allele Frequencies
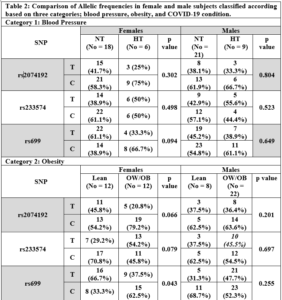
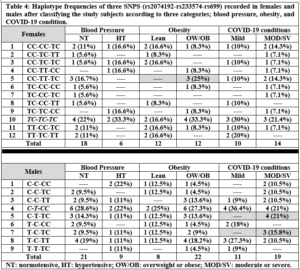
After reading all sequencing chromatograms, alleles for all three SNPs were counted and recorded for either females or males included in any of the three categories; blood pressure, obesity, and COVID-19 condition. Of note, and since ACE2 gene occurs on X chromosome whereas AGT occurs on an autosome, for both rs2074192 and rs233574 we counted two alleles in females but only one allele in males, while for rs699 we counted the two alleles in both females and males. Table 2 shows the number of alleles in females and males recorded for each of the three SNPs, and according to the distribution of the study subjects in the three categories. The only recorded significant difference was in rs699 when comparing the T and C allele frequencies between lean versus OW/OB females, where the C allele was significantly higher in OW/OB females compared to lean females, p=0.043.
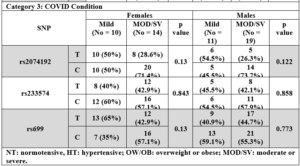
Noteworthy, a higher rs699 C allele frequency was recorded in HT compared to NT female subjects, a higher rs2074192 C allele frequency was recorded in OW/OB compared to lean subjects, a higher rs233574 C allele frequency was recorded in lean compared to OW/OB subjects, and a higher rs2074192 C allele frequency was recorded in both female and male subjects with MOD/SV compared to mild COVID-19 conditions, although none of the comparisons reached significance, p>0.05.
Genotype Frequencies
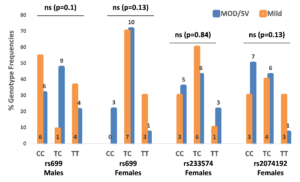
Genotypes were counted and recorded for all three SNPs. In all three categories, no significant differences were detected vis-à-vis genotype frequencies among NT and HT, lean and OW/OB, or mild and MOD/SV phenotypes. Fig. 2 shows genotype frequencies in the COVID-19 condition category for rs699 in both females and males, while it shows genotype frequencies for rs2074192 and rs233574 only in females. Since ACE2 occurs on the X-chromosome, the genotypes for either rs2074192 or rs233574 in males are the same as the allele frequencies shown in Table 2. Of note, the rs2074192 CC genotype appeared in 7 out of 14 female subjects with MOD/SV condition (50%) compared to 3 out of 10 female subjects (30%) with mild condition; the rs699 CC genotype appeared in 3 out of 14 female subjects with MOD/SV condition (21.5%) while no female had this genotype in the mild condition population; and finally the rs699 TC genotype appeared in 9 out of 19 male subjects with MOD/SV condition (47.4%) while it appeared in only 1 male out of 11 male subjects (9.1%) with mild condition.
Haplotype Frequencies
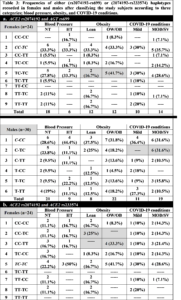
The distribution of possible haplotypes including rs2074192, rs233574, and rs699 in either female or male subjects was counted and their frequencies recorded (Tables 3 & 4). Table 3 shows the haplotype frequencies when only two SNPs are taken separately; i.e. rs2074192 and rs699, or rs2074192 and rs233574, while Table 4 shows the haplotype frequencies for all three SNPs together.
ACE2 rs2074192 – AGT rs699 Haplotypes
Table 3(a) shows all 9 possible haplotypes (32) for females and all 6 haplotypes for males. In females, the most common haplotype when considering both rs2074192 and rs699 polymorphisms was CC-TC, respectively. Among the three categories, the highest difference in haplotype frequencies was detected for TC-TC haplotype between the lean (16.7%) and OW/OB (41.7%) groups. The TT-CC haplotype was not recorded in female subjects. In males, the highest recorded haplotype for rs2074192 and rs699 was C-CC, respectively. Moreover, 6 out of 19 male subjects with MOD/SV condition had the C-TC haplotype, while no males out of 11 were recorded with this haplotype in male subjects with mild conditions. Similarly, 3 OW/OB male subjects had the C-TT haplotype, while no lean males were recorded with this haplotype.
ACE2 rs2074192 – ACE2 rs233574 Haplotypes
Table 3(b) shows all 9 possible haplotypes (32) for females and all 4 haplotypes for males. In females, the most common haplotype when considering both rs2074192 and rs233574 genotypes was TC-TC, respectively. In addition, 4 females (33.3%) had the CC-TT haplotype in the OW/OB groups, while no females had this haplotype in the lean group. Reversibly, 3 females (25%) had the CC-TC haplotype in the lean groups, while no females had this haplotype in the OW/OB group. In males, the highest recorded haplotype was C-T. Moreover, the highest difference among haplotype frequencies was detected for C-C haplotype in the mild (1 male, 9.1%) and MOD/SV (6 males, 31.6%) groups.
ACE2 rs2074192 – ACE2 rs233574 – AGT rs699 Haplotypes
Table 4 shows the 12 recorded haplotypes out of the 27 (33) possible haplotypes in females and 9 recorded haplotypes out of 12 possible haplotypes for males. In females, the most frequent haplotype among the three categories was TC-TC-TC. In addition, 3 females (25%) in the OW/OB group showed the CC-TT-TC haplotype, while no females showed this haplotype in the lean group. Contrariwise, 3 females (16.7%) in the NT group showed the CC-TT-TC haplotype, while no females showed this haplotype in the HT group. One female had the CC-TT-CC haplotype in all three groups: HT, OW/OB and MOD/SV. Of note, 15 possible haplotypes were absent in females including TT-CC-TT. In males, the highest recorded haplotype in the three categories was C-T-CC. In addition, the highest differences among haplotype frequencies were detected for C-T-TC and T-C-TC haplotypes, where 4 males (21%) in the MOD/SV groups have C-T-TC in comparison with none in the mild groups, and 3 males (15.8%) in the MOD/SV groups have T-C-TC in comparison with none in the mild groups.
Finally, among the MOD/SV group, three females aged (55, 76 and 86), and with BMI (25, 34, and 26) had a life-threatening condition that required their admission to the ICU at a local hospital. The haplotypes of the four subjects were (TC-TC-TC), (CC-CC-TC), (CC-TT-CC).
DISCUSSION
Age, Obesity, and Sex of Study Subjects
We recorded no significant differences in mean age or BMI between the 24 females and the 30 males included in this study. However, normotensive subjects were clearly younger and leaner than hypertensive subjects (Table 1). In fact, it is well known that aging and obesity are independent risk factors for raised blood pressure levels [25,26]. Moreover, it has been reported that overweight occurring at a younger age at onset is associated with a significantly increased risk of hypertension with the highest relative risk among individuals aged 18–39 years as onset of overweight [27]. As for COVID-19 severity, subjects with moderate and severe conditions were significantly older but not more obese than those with mild conditions (Table 1). In fact, the severity of COVID-19 is known to be associated with specific pre-existing conditions including age, sex, obesity, hypertension and genetic susceptibility, with mortality occurring mostly in the elderly and being about twice as high in males as in females [18,28]. Although no significant difference in BMI was found between subjects with mild compared to MOD/SV conditions (Table 1), it is noteworthy that 22 out of 30 males (73.3%) and 12 out of 24 females (50%) were overweight or obese (Table 2). This made the majority of subjects enrolled in this study as OW/OB, with the mean BMI in this group (26.9 kg/m2) already in the overweight range, and probably justifies the absence of differences in BMI between subjects with mild and MOD/SV COVID-19 conditions. Of note, no differences were found regarding sex distribution after classification of study subjects according to blood pressure and COVID-19 severity (Table 2); females (NT 75%, HT 25%; Mild 41.7%, MOD/SV 58.3%) and males (NT 70%, HT 30%; Mild 36.7%, MOD/SV 63.3%).
ACE2 rs2074192 C>T
The rs2074192 polymorphism is a variation in intron 16 of the ACE2 gene located on the X chromosome, with a global C allele frequency around 56.9% and a T allele frequency around 43.1%, European (C 55.3%, T 44.7%), African (C 68.4%, T 31.6%), and Asian (C 57.9%, T 42.1%), (www.snpedia.com). Pouladi and Abdolahi showed in silico a major effect of this SNP on the secondary structure of ACE2 RNA [23], suggesting a possible dysregulation of ACE2 transcription or translation and protein stability, and leading to a decrease in SARS-CoV-2 viral entry. In fact, many previous reports have recorded an association, although controversial, between rs2074192 with hypertension and COVID-19 severity [11,17,19,20,29]. In the Chinese population, Luo et al. demonstrated that the rs2074192 T allele was associated with essential hypertension in Chinese patients [11]. Moreover, Pan et al. reported that individuals with TC or TT genotype were associated with essential hypertension [29]. Sheikhian et al. showed that the ACE2 rs2074192 TT genotype was associated with the COVID-19 mortality in Iranian COVID-19 patients [20]. On the contrary, in Mexican patients, Martinez-Gomez et al. reported a higher C allele frequency and CC genotype frequency in COVID-19 patients with severe and critical conditions [17]. Similarly, Molina et al. showed that the T allele was protective against COVID-19 severity in a cohort of Italian patients [19]. These previous reports highlight the differences in genetic composition among different ethnicities and raise the importance of examining SNP distribution among other populations, including Syrians. Despite the fact that no significant differences were found in the current study when comparing rs2074192 allelic and genotypic frequencies between female and male subjects, the data suggest a modest association between the C allele with hypertension, obesity and COVID-19 severity as allele frequencies were higher in hypertensive females compared to normotensive peers (p value = 0.3), in OW/OB females compared to lean subjects (p value = 0.066), and in female and male subjects with MOD/SV conditions compared to those with mild conditions (p value = 0.13, and 0.12, respectively) (Table 2). In addition, the CC genotype frequency was higher in female subjects with MOD/SV compared to those with mild conditions (Fig. 2). These data are consistent with a protective effect of the T allele possibly by reducing the effect of the ACE2 enzyme that hydrolyzes Ang-II and prevents the latter’s contribution to vasoconstriction, fibrosis, inflammation, and thrombosis [8], whereas the C allele or CC genotype could be considered as risk factors.
ACE2 rs233574 T>C
The rs233574 polymorphism is an intronic variation in the ACE2 gene on the X-chromosome, with a global T allele frequency around 51.8% and C allele frequency around 48.2%, European (T 50.5%, C 49.5%), African (T 69.1%, C 30.9%), and Asian (T 30%, C 70%), (www.snpedia.com). Similar to rs2074192, the rs233574 theoretically affects the RNA secondary structure possibly leading to destabilizing the ACE2 transcript and protein [22,23]. Nevertheless, the T reference allele may additionally enhance the affinity for binding ETR-3 splicing factor, thus increasing susceptibility for COVID-19 [30]. Interestingly, most ethnic populations have comparably mid or low C allele frequency, while its frequency in the Chinese population could reach 70%, hence this allele could have been played its protective role in a large number of Chinese COVID-19 patients, although no clinical data are available to support the protective role of rs233574. Likewise, data from our study might support a protective effect for the C allele, while the T allele is a risk factor. In fact, higher T allele frequencies were detected in hypertensive females (50%) and males (55.6%) compared to normotensive peers (38.9% and 42.9%, respectively), although with no significance (p value > 0.05), and in OW/OB compared to lean female subjects (p value = 0.079), while very similar C and T allele frequencies were found in female and male subjects with MOD/SV compared to those with mild conditions (p value = 0.85) (Table 2). Finally, the TT genotype frequency was higher in female subjects with MOD/SV compared to those with mild conditions (Fig. 2).
AGT rs699 T>C
This polymorphism occurs on exon 2 in the AGT gene on chromosome 1, leading to replacement of a methionine residue with threonine in the 268 position within the primary protein structure, with a global T allele frequency around 54.5% and a C allele frequency around 45.5%, European (T 57.9%, C 42.1%), African (T 18.1%, C 81.9%), and Asian (T 17.5%, C 82.5%), (www.snpedia.com). In fact, the rs699 C allele has been reported to be associated with increased plasma angiotensinogen levels, leading to hypertension [7]. Several previous reports showed a significant association of rs699 with hypertension and/or COVID-19 severity [24,31-35]. Specifically, Mirahmadi et al. found that the rs699 C allele is a predisposing variant for coronary artery disease (CAD) in the Iranian population [32]; Repchuk et al. found that the rs699 C allele was associated with a 2.5-fold increase in systolic and diastolic BP in Ukrainian patients [33]; Yako et al. found an association between rs699 and hypertension in Tunisian and South African patients [34]; Cafiero et al. found a higher allele C (45%) and CC genotype (22%) frequencies in symptomatic compared to asymptomatic (C 31%, CC 0%) Italian COVID-19 patients [31]; and Kouhpayeh et al. demonstrated that the TC genotype and the C allele of AGT rs699 increased the risk of COVID-19 infection in Iranian COVID-19 patients [24]. Finally, Khamlaoui et al., found that rs699 was associated with high BMI, waist circumference and overweight/obesity [35]. Data from our current study demonstrated the presence of a higher rs699 C allele frequency in hypertensive compared to normotensive, OW/OB compared to lean, and MOD/SV compared to mild COVID-19, although with larger differences in females compared to males, and reaching significance only when comparing OW/OB females with lean females, p=0.043, (Table 2). In addition, the CC genotype appeared in 3 females in the MOD/SV group compared to none in the Mild groups (Fig 2). Hence, from these data, rs699 C could be extrapolated as a risk allele while T as a protective allele in relation to COVID-19 severity. Notably, all subjects included in our study were infected by COVID-19, although with a range of symptoms. Hence, comparing the allele frequencies in our subjects for all three SNPs with those previously reported in different ethnicities and populations would probably be imprecise, especially when taking into account the large differences in allele frequencies among different populations, as in the case of rs233574 and rs699. Nevertheless, when considering the allele frequency values in normotensive female subjects (Table 2), we could find out allelic frequencies of rs2074192 similar to those in most ethnicities, allelic frequencies of rs233574 similar to those in Asians but not in Europeans and Africans, and slightly higher allelic frequencies of rs699 compared to those in Europeans, but soundly different from their frequencies in Africans and Asians. In fact, the deviation of allelic frequency in the Syrian population documented in this study is not surprising taking into consideration the geographical location of Syria amid the three continents, where the genetic composition of Syrians is expected to be unique compared to most other populations because of migration and breeding influenced by several factors over thousands of years. Nonetheless, a future study reporting the allelic frequencies of all three SNPs in healthy Syrian controls would result in accurate estimates of SNP frequencies. On the other hand, it was interesting to note that the differences in allelic frequencies for all three SNPs were significant or close to significance with small p-values, only when comparing these frequencies in the obesity but not in the blood pressure and COVID-19 condition categories, specifically between lean and OW/OB female subjects (Table 2). In fact, it is very well documented that visceral obesity is a risk factor for cardiovascular disease, metabolic syndrome, hypercoagulability and vitamin D deficiency, all of which are hallmarks for COVID-19 severity [15,36,37]. Recently, Steenblock et al., identified several factors involved in the mechanism by which obesity affects COVID-19 outcome, including physical stress on ventilation, increased risk of pulmonary fibrosis, impairment of the immune system and accumulation of macrophages in adipose tissues, increasing adiponectin levels that can stimulate macrophages production of pro-inflammatory cytokines such as TNF-α, IL-6 and IL-1β, and finally activating the RAAS leading to increased blood pressure, atherosclerosis and thrombosis [38]. One could postulate that possible effects of rs2074192, rs233574, and rs699 on either blood pressure levels and/or COVID-19 severity could be occurring via indirect involvement of obesity-dependent biochemical pathways. This could be further tested on a large number of individuals who suffered from severe COVID-19 disease. Equally remarkable are the sound differences in the allele frequency profiles for all three SNPs between females and males within the obesity category (Table 2), in addition to the different distribution of rs699 genotypes between females and males within the COVID-19 category (Fig. 2). Actually, similar differences were previously reported not only for X-linked gene polymorphisms, but even for genes located on autosomes. For example, Hamet et al. showed that the T allele of rs2074192 was associated with hypertension only in obese males, while reporting a significant interaction with obesity of another SNP in the ACE2 gene, rs233575, but only in females [18]. On the other hand, Repchuk et al. showed that the rs699 C allele was associated with high systolic and diastolic blood pressure only in females [33]. As a matter of fact, sex related genotype-phenotype interactions are highly complicated and was previously tackled by many researchers. Indeed, endocrine status, in addition to genetic composition, vitally affects phenotypes and could be influenced by several factors, including differences in hormonal levels and even lifestyle habits, such as smoking and alcohol consumption, etc [39-41].
Haplotypes
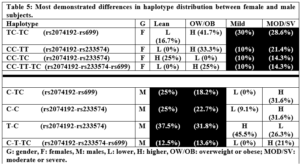
Similar to the aforementioned differences in allelic and genotypic frequencies among females and males, distribution of haplotypes varied between the two sexes (Tables 3 & 4). This was clear in the different distribution of the most common haplotypes in females and males; for rs2079142-rs699, CC-TC in females, and C-CC in males; for rs2079142-rs233574, TC-TC in females and C-T in males; and for rs2079142-rs233574-rs699, TC-TC-TC in females and C-T-CC in males. This could certainly contribute to the phenotypic differences between the two sexes and explain the distinct association of certain alleles, genotypes, and haplotypes with blood pressure, obesity, and COVID-19 severity. Table 5 summarises the clearest differences in haplotype distribution among the two sexes demonstrated in Tables 3 and 4.
A review of Table 5 highlights one important finding: different haplotypes were associated with obesity only in female subjects, whereas other haplotypes were associated with COVID-19 conditions only in male subjects. Once again, this eludes to the disparity between the two sexes in terms of genetic composition and its phenotypic effects. Several preliminary conclusions might be drawn from Table 5: the rs2074192 CC genotype is probably associated with obesity in females; the rs699 TC genotype is probably not associated with either obesity or COVID-19 severity, as it was commonly distributed among females and males; the rs233574 TT or TC genotypes are probably associated with obesity; the rs2074192 C allele is associated with obesity in females and COVID-19 severity in males; and the rs2074192 T allele could be protective. Nonetheless, further studies should be performed to check for the validity of these proposals. Finally, two haplotypes were overrepresented in the HT compared to NT subjects (Table 3); the rs2074192-rs699 C-CC haplotype in males, the rs2074192-rs233574 TC-TC haplotype in females. In both cases, this could be due to the presence of the rs2074192 C allele that has previously been reported to be associated with hypertension in COVID-19 patients [17,19]. Taken together, and suggesting that the rs2074192 C allele, the rs233574 T allele, and the rs699 C allele, as risk alleles for COVID-19 morbidity and/or comorbidities, one could hypothesize that the most risky haplotype would be CC-TT-CC in females, and C-T-CC in males, while the most protective haplotype would be TT-CC-TT in females, and T-C-TT in males. Indeed, the fact that we recorded only 12 out of 27 possible haplotypes in our female population (Table 4), while the rest 15 unfound haplotypes included the TT-CC-TT haplotype might support our proposed haplotype functional classification. On the contrary, life-threatening cases could have haplotypes identical or similar to the risky haplotypes we proposed. In fact, three cases in our study population had life-threatening conditions that required them to be admitted to the ICU at local hospitals. The haplotypes of these three female subjects were (TC-TC-TC), (CC-CC-TC), (CC-TT-CC), i.e., one identical and two close haplotypes to the proposed most risky haplotype CC-TT-CC. Nonetheless, the three female subjects were elderly with ages (55, 76 and 86) and BMI (25, 34, and 26), so it is not possible to isolate the possible effect of age on COVID-19 severity in these individuals.
CONCLUSIONS
In this study, the allele, genotype and haplotype frequencies of three single nucleotide polymorphisms, ACE2 rs2074192, ACE2 rs233574, and AGT rs699 were recorded in a cohort of Syrian subjects who had previously suffered from COVID-19 disease with variable symptoms and comorbidities. We found significant association between rs699 and obesity in the female subject, while the other two SNPs showed reasonable association but without statistical significance. Based on our data and those from previous reports, we proposed the C allele for rs2074192, the T allele for ACE2 rs233574, and the C allele of AGT as risk factors for obesity and COVID-19 severity, although the association with obesity was shown only in female subjects while the association with COVID-19 severity was shown only in male subjects. We also proposed two haplotypes, rs2074192-rs233574-rs699 (CC-TT-CC in females, and C-T-CC) as risky combination for COVID-19 severity, while two haplotypes (TT-CC-TT in females, and T-C-TT in males) as protective. Our study has two main limitations; the first is the small number of studied subjects, which might have obscured significant associations. The second limitation is related to the retrospective nature of our study, which did not allow us to follow the clinical outcomes of the subjects.
ACKNOWLEDGEMENT
The authors thank Ms. Reham Antaki for her superb technical assistance.
References :- Ragia G, and Manolopoulos VG. Assessing COVID-19 susceptibility through analysis of the genetic and epigenetic diversity of ACE2 mediated SARS-CoV-2 entry. 2020 Dec; 21(18):1311-1329. doi: 10.2217/pgs-2020-0092.
- Asselta R, Paraboschi EM, Mantovani A, Duga S. ACE2 and TMPRSS2 variants and expression as candidates to sex and country differences in COVID-19 severity in Italy. Aging (2020); 12:10087-98.
- Choudhary S, Sreenivasulu K, Mirta P, et al. Role of genetic variants and gene expression in the susceptibility and severity of COVID-19. Ann Lab Med, 2021; 41:129-138.
- Niemi MEK, Daly MJ, and Ganna A. The human genetic epidemiology of COVID-19. Nature Reviews /Genetics/, 2022, 23: 533-546. https://doi.org/10.1038/ s41576-022-00478-5.
- Saengsiwaritt W, Jittikoon J, Chaikledkaew U and Udomsinprasert W. Genetic polymorphisms of ACE1, ACE2, and TMPRSS2 associated with COVID-19 severity: A systematic review with meta-analysis. Rev Med Virol. 2022 Jul; 32(4):e2323. doi: 10.1002/rmv.2323.
- Carluccio, M, M Soccio, and R De Caterina. “Aspects of Gene Polymorphisms in Cardiovascular Disease: The Renin-Angiotensin System(s) (RAS).” Eur J Clin Invest., 2001; Vol. 31.
- Herrera CL, Castillo W, Estrada P, et al. Association of polymorphisms within the Renin-Angiotensin System with metabolic syndrome in a cohort of Chilean subjects. Arch Endocrinol Metab., 2016; 60/3. DOI: 10.1590/2359-3997000000134.
- Kanugula, Ashok Kumar, Jasleen Kaur, Jaskaran Batra, Anvitha R Ankireddypalli, and Ravikanth Velagapudi. Renin-Angiotensin System: Updated Understanding and Role in Physiological and Pathophysiological States. Cureus, June 2023. https://doi.org/10.7759/cureus. 40725.
- Liu D, Chen Y, Zhang P, et al. Association between circulating levels of ACE2-Ang-(1–7)-MAS axis and ACE2 gene polymorphisms in hypertensive patients. Medicine, 2016, 95:24(e3876).
- Yang Y, Song Y, and Hou D. Obesity and COVID-19 Pandemics: Epidemiology, Mechanisms, and Management. Diabetes, Metabolic Syndrome and Obesity 2023:16 4147–4156. https://doi.org/10.2147/DMSO.S441762.
- Luo Y, Liu C, Guan T, et al. Association of ACE2 genetic polymorphisms with hypertension related target organ damages in south Xinjiang. Hypertension Research, 2019, 42:681–689 https://doi.org/10.1038/s41440-018-0166-6F.
- Bosso M, Alphonse Thanaraj T, Abu-Farha M. et al. The Two Faces of ACE2: The Role of ACE2 Receptor and Its Polymorphisms in Hypertension and COVID-19. Molecular Therapy: Methods & Clinical Development, 2020: Vol. 18 September. https://doi.org/10.1016/j.omtm. 2020.06. 017.
- Deng H, Yan X, and Yuan L. Human genetic basis of coronavirus disease 2019.
Signal Transduction and Targeted Therapy, 2021; 6: 344; https://doi.org/10.1038/s41392-021-00736-8. - Fiore JR, Di Stefano M, Oler A, et al. Lack of Evidence for a Role of ACE-2 Polymorphisms as a Bedside Clinical Prognostic Marker of COVID-19. Viruses, 2023; 15, 1448. https://doi.org /10.3390/v15071448.
- Horowitz JE, Kosmicki JA, Damask D, et al. Genome-wide analysis provides genetic evidence that ACE2 influences COVID-19 risk and yields risk scores associated with severe disease. Nature Genetics, 2022; 54:382–392. https://doi.org/10.1038/s41588-021-01006-7.
- Feng S, Song F, Guo W, et al. Potential Genes Associated with COVID-19 and Comorbidity. International Journal of Medical Sciences, 2022; 19(2): 402-415. doi: 10.7150/ijms.67815
- Martinez-Gomez LE, Herrera-Lopez B, Martinez-Armeta C, et al. ACE and ACE2 Gene Variants Are Associated With Severe Outcomes of COVID-19 in Men. Frontiers in Immunology, 2022. doi: 10.3389/fimmu.2022.812940.
- Hamet P, Pausova Z, Attaoua R, et al. SARS–CoV-2 Receptor ACE2 Gene Is Associated with Hypertension and Severity of COVID 19: Interaction with Sex, Obesity, and Smoking. American Journal of Hypertension, 2021; 34(4). doi:10.1093/ajh/hpaa223.
- Molina MS, Rocamora ER, Bendicho A, et al. Polymorphisms in ACE, ACE2, AGTR1 genes and severity of COVID-19 disease. PLOS ONE February 4, 2022. https://doi.org/10.1371/pone.0263140.
- Sheikhian F,Mofrad SS,Tarashi S,et al.The impact of ACE2 polymorphisms (rs1978124, rs2285666, and rs2074192) and ACE1 rs1799752 in the mortality rate of COVID‑19 in different SARS‑CoV‑2 variants. Human Genomics, 2023; 17:54. https://doi.org/10.1186/s40246-023-00501-8.
- Suleiman AA, Rafaa TA, Alrawi AM, and Dawood MF. The impact of ACE2 genetic polymorphisms (rs2106809 and rs2074192) on gender susceptibility to COVID-19 infection and recovery: A systematic review. Baghdad Journal of Biochemistry and Applied Biological Sciences, 2021; 2(03):167-180. doi: bjbabs.v2i03.53.
- Jami G, Ataee M, Esmaeili V, et al. Characterization of the angiotensin-converting enzyme 2 (ACE2), the main receptor for the SARS-CoV-2 virus. Am J Clin Exp Immunol 2023; 12(3):24-44.
- Pouladi N, and Abdolahi S. Investigating the ACE2 polymorphisms in COVID-19 susceptibility: An in silico analysis. Mol Genet Genomic Med. 2021; 9:e1672. https://doi.org/10.1002/mgg3.1672.
- Kouhpayeh HR, Tabasi F, Dehvari M, et al. Association between angiotensinogen (AGT), angiotensin-converting enzyme (ACE) and angiotensin-II receptor 1 (AGTR1) polymorphisms and COVID-19 infection in the southeast of Iran: a preliminary casecontrol study. Translational Medicine Communications, 2021; 6:26 https://doi.org/10.1186/s41231-021-00106-0.
- Jayedi A, Rashidy-Pour A, Khorshidi M, et al. Body mass index, abdominal adiposity, weight gain and risk of developing hypertension: a systematic review and dose-response meta-analysis of more than 2.3 million participants. Obes Rev, 2018; 19:654–67. doi: 10.1111/obr.12656.
- Thompson P, Logan I, Tomson C, Sheerin N, and Ellam T. Obesity, Sex, Race, and Early Onset Hypertension Implications for a Refined Investigation Strategy. Hypertension, 2020; 76:859-865. DOI: 10.1161/HYPERTENSIONAHA.120.15557.
- Li W, Fang W, Huang Z, et al. Association between age at onset of overweight and risk of hypertension across adulthood. Heart, 2022; 108:683–688. doi:10.1136/heartjnl-2021-320278.
- Hippisley-Cox J, Coupland CA, Mehta N, et al. Risk prediction of covid-19 related death and hospital admission in adults after covid-19 vaccination: national prospective cohort study. BMJ. 2021; 374:n2244. doi:10.1136/bmj.n2244.
- Pan Y, Wang T, Li Y, et al. Association of ACE2 polymorphisms with susceptibility to essential hypertension and dyslipidemia in Xinjiang, China. Lipids in Health and Disease, 2018; 17:241. https://doi.org/10.1186/s12944-018-0890-6.
- Delanghe JR, and Speeckaert MM. Host polymorphisms and COVID-19 infection. Advances in Clinical Chemistry, 2022; Volume 107. https://doi.org/10.1016/bs.acc.2021.07.002.
- Cafiero C, Rosapepe F, Palmirotta R, et al. Angiotensin System Polymorphisms’ in SARS-CoV-2 Positive Patients: Assessment Between Symptomatic and Asymptomatic Patients: A Pilot Study. Pharmacogenomics and Personalized Medicine, 2021; 1:14 621–629. DOI https://doi.org/ 10.2147/PGPM.S303666.
- Mirahmadi M, Salehi A, Golalipour M, Bakhshandeh A, and Shahbazi A. Association of rs5051 and rs699 polymorphisms in angiotensinogen with coronary artery disease in Iranian population A case-control study. Medicine, 2024; 103:11(e37045). http://dx.doi.org/10.1097/MD.00000 00000037045.
- Repchuk Y, Sydorchuk LP, Sydorchuk AR, et al. Linkage of blood pressure, obesity and diabetes mellitus with angiotensinogen gene (AGT 704T>C/rs699) polymorphism in hypertensive patients. Bratisl Med J, 2021; 122 (10). DOI: 10.4149/BLL_2021_114.
- Yako YY, Balti EV, Matsha TE, et al. Genetic factors contributing to hypertension in African-based populations: A systematic review and meta-analysis. J Clin Hypertens. 2018; 20:485–495. DOI: 10.1111/jch.13225.
- Khamlaoui W, Mehri S, Hammami S, Elosua R, and Hammami M. Association of angiotensin-converting enzyme insertion/deletion (ACE I/D) and angiotensinogen (AGT M235T) polymorphisms with the risk of obesity in a Tunisian population. Journal of the Renin-Angiotensin-Aldosterone System, 2020; April-June, 1–7. https://doi.org/10.1177/ 14703203209078.
- Golzarand M, Hollis BW, Mirmiran P, et al. Vitamin D supplementation and body fat mass: a systematic review and meta-analysis. Eur J Clin Nutr, 2018; 72: 1345–1357.
- Russo L, Lumeng CN. Properties and functions of adipose tissue macrophages in obesity. Immunology 2018; 155: 407–417.
- Steenblock C, Hassanein M, Khan EG, et al. Obesity and COVID-19: What are the Consequences? Horm Metab Res, 2022; 54: 496–502. DOI 10.1055/a-1878-9757.
- Foresta C, Rocca MS, Di Nisio A. Gender susceptibility to COVID‑19: a review of the putative role of sex hormones and X chromosome. Journal of Endocrinological Investigation, 2021; 44:951–956.
- Gagliardi MC, Tieri P, Ortona E, and Ruggieri A. ACE2 expression and sex disparity in COVID-19. Cell Death Discovery, 2020; 6:37. https://doi.org/10.1038/s41420-020-0276-1.
- Li Y, Jerkic M, Slutsky AS, and Zhang H. Molecular mechanisms of sex bias differences in COVID-19 mortality. Critical Care, 2020; 24:405. https://doi.org/10.1186/s13054-020-03118-8.
The Authors declare that they have no competing interests.
Funding: No Fund.
Data and materials availability: All data are available in the main text.
(ISSN - Online)
2959-8591